Exam 2: Atoms, molecules, and Ions
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
How many of the following postulates of Dalton's atomic theory are still scientifically accepted?
I.All atoms of the same element are identical.
II.Compounds are combinations of different atoms.
III.A chemical reaction changes the way atoms are grouped together.
IV.Atoms are indestructible.
(Multiple Choice)
4.9/5  (39)
(39)
The Greeks proposed that matter consisted of four fundamental substances:
(Multiple Choice)
4.8/5  (38)
(38)
The chemist credited for inventing a set of symbols for writing elements and a system for writing the formulas of compounds (and for discovering selenium,silicon,and thorium)is
(Multiple Choice)
4.8/5  (43)
(43)
Arsenopyrite is a mineral containing As,Fe,and S.Classify each element as metal,nonmetal,or metalloid.
(Essay)
4.8/5  (32)
(32)
The number of neutrons in an atom is the same for all neutral atoms of that element.
(True/False)
5.0/5  (30)
(30)
All of the following are characteristics of nonmetals except:
(Multiple Choice)
4.8/5  (32)
(32)
Rutherford's experiment was important because it showed that:
(Multiple Choice)
4.7/5  (39)
(39)
The scientist who discovered the law of conservation of mass and is also called the father of modern chemistry is
(Multiple Choice)
4.8/5  (37)
(37)
Three samples of a solid substance composed of elements A and Z were prepared.The first contained 4.31 g A and 7.70 g Z.The second sample was 35.9% A and 64.1% Z.It was observed that 0.718 g A reacted with Z to form 2.00 g of the third sample.Show that these data illustrate the law of definite composition.
(Essay)
4.7/5  (34)
(34)
Consider the following two compounds: H2O 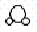 and H2O2
and H2O2 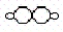 .According to the law of multiple proportions,the ratio of hydrogen atoms per gram of oxygen in H2O to hydrogen atoms per gram of oxygen in H2O2 is
.According to the law of multiple proportions,the ratio of hydrogen atoms per gram of oxygen in H2O to hydrogen atoms per gram of oxygen in H2O2 is
(Multiple Choice)
4.9/5  (36)
(36)
Showing 61 - 80 of 91
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)