Exam 26: Structures of Organic Compounds
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
Choose the best configuration assignments for the three double bonds, labeled A, B and C, in the following compound: 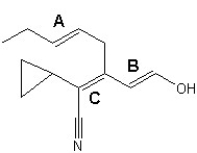
(Multiple Choice)
4.8/5  (37)
(37)
A solid wedge line denotes a bond that sticks back behind the plane of the paper.
(True/False)
4.8/5  (39)
(39)
What is the degree of unsaturation for the following compounds? 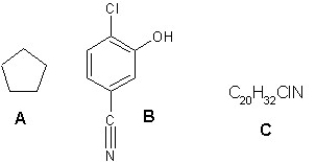
(Multiple Choice)
4.8/5  (41)
(41)
The two benzene substituents of the common analgesic shown below are considered in what relation to one another? 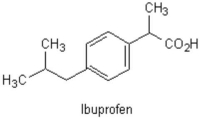
(Multiple Choice)
4.9/5  (48)
(48)
Constitutional isomers have different bond connectivities, and as a consequence they have different skeletal structures.
(True/False)
4.8/5  (35)
(35)
Which of the following has the lowest melting point: cyclohexane, hexane, benzene?
(Multiple Choice)
4.8/5  (33)
(33)
How many different structural isomers are there of the compound dichlorobutane?
(Multiple Choice)
4.8/5  (33)
(33)
Can 1,3,5-hexatriene be considered an aromatic compound? Why do you think so?
(Multiple Choice)
4.7/5  (41)
(41)
What is the correct IUPAC name for the following structure? 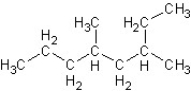
(Multiple Choice)
4.7/5  (36)
(36)
Compound A easily reacts with water to give compound B. Compound B on the other hand can be oxidized to produce compound C. Both B and C can be further oxidized to produce D. To which classes of organic compounds A, B, C and D most likely belong?
(Multiple Choice)
4.8/5  (41)
(41)
What is the complete systematic name for the following molecule? 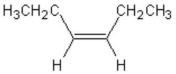
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following five positions would result in the most stable molecule for a large group like t-butyl, -C(CH3)3?
(Multiple Choice)
4.9/5  (42)
(42)
Calculate the degree of unsaturation for the molecule below: 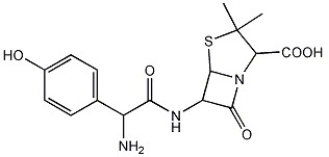
(Multiple Choice)
4.7/5  (43)
(43)
The burning of alkanes to produce carbon dioxide and water is known as what type of reaction?
(Multiple Choice)
4.8/5  (31)
(31)
Showing 41 - 60 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)