Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
The chemistry of Be is different from that of the other group 2 metals due to the small size of Be2+.
(True/False)
4.9/5  (32)
(32)
Choose the INCORRECT statement about the alkaline earth metals.
(Multiple Choice)
4.9/5  (34)
(34)
The Ka for boric acid is 5.6 × 10-10. What is the pH for 0.10 sodium borate?
(Multiple Choice)
4.9/5  (32)
(32)
Bronze is 90% Cu and 10% Sn by mass. What is the density of bronze if the density of Cu is 8.95 g/cm3 and the density of Sn is 5.77 g/cm3?
(Multiple Choice)
4.9/5  (32)
(32)
The production of lead is a two step process:
2 PbS(s) + 3 O2(g) 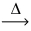 2 PbO(s) + 2 SO2(g)
2 PbO(s) + C(s)
2 PbO(s) + 2 SO2(g)
2 PbO(s) + C(s) 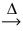 2 Pb(l) + CO2(g)
How much ore that is 5.2% PbS is necessary to produce 12.0 kg of Pb?
2 Pb(l) + CO2(g)
How much ore that is 5.2% PbS is necessary to produce 12.0 kg of Pb?
(Multiple Choice)
4.9/5  (31)
(31)
Choose the INCORRECT statement about the inert-pair effect.
(Multiple Choice)
4.7/5  (35)
(35)
A sample of water has hardness expressed as 81 ppm Ca2+. The sample is passed through an ion exchange column and the Ca2+ ions are replaced by H+ ions. What is the pH of the water after it has been treated? (1 ppm = 1 g solute/106 g solution)
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following is INCORRECT? A reaction typical for an alkali metal (M) is:
(Multiple Choice)
4.9/5  (32)
(32)
The difference between temporary hard water and permanent hard water is that only the former contains a significant concentration of:
(Multiple Choice)
4.8/5  (36)
(36)
Showing 81 - 100 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)