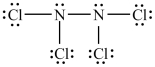Exam 4: Covalent Compounds
Exam 1: Matter and Measurement89 Questions
Exam 2: Atoms and the Periodic Table90 Questions
Exam 3: Ionic Compounds90 Questions
Exam 4: Covalent Compounds90 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: Energy Changes, Reaction Rates, and Equilibrium88 Questions
Exam 7: Gases, Liquids, and Solids79 Questions
Exam 8: Solutions90 Questions
Exam 9: Acids and Bases90 Questions
Exam 10: Nuclear Chemistry85 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups103 Questions
Exam 12: Alkanes106 Questions
Exam 13: Unsaturated Hydrocarbons101 Questions
Exam 14: Organic Compounds That Contain Oxygen, Halogen, or Sulfur111 Questions
Exam 15: The Three-Dimensional Shape of Molecules100 Questions
Exam 16: Aldehydes and Ketones103 Questions
Exam 17: Carboxylic Acids, Esters, and Amides81 Questions
Exam 18: Amines and Neurotransmitters105 Questions
Exam 19: Lipids105 Questions
Exam 20: Carbohydrates92 Questions
Exam 21: Amino Acids, Proteins, and Enzymes88 Questions
Exam 22: Nucleic Acids and Protein Synthesis89 Questions
Exam 23: Digestion and the Conversion of Food Into Energy92 Questions
Exam 24: Carbohydrate, Lipid, and Protein Metabolism91 Questions
Select questions type
In the valence shell electron pair repulsion (VSEPR)theory, a group is defined as an atom or a lone pair of electrons.
Free
(True/False)
4.9/5  (46)
(46)
Correct Answer:
True
Carbon tetrachloride has _____ valence electrons.
Free
(Short Answer)
4.7/5  (41)
(41)
Correct Answer:
thirty-two or 32
The Lewis structure shown below is not a valid Lewis structure. What statement best describes the error in the structure? 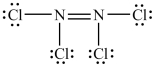
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following is classified as a group in the valence shell electron pair repulsion (VSEPR)theory?
(Multiple Choice)
4.8/5  (35)
(35)
The symbol - is given to the more electronegative atom in a polar bond.
(True/False)
4.9/5  (34)
(34)
Unequal sharing of electrons in a covalent bond results in a polar bond.
(True/False)
4.8/5  (35)
(35)
The Lewis structure for PH3 contains an atom that does not follow the octet rule.
(True/False)
4.7/5  (49)
(49)
When writing Lewis structures, the symbol below is placed between resonance structures. 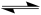
(True/False)
4.9/5  (34)
(34)
Which molecule's Lewis structure contains an atom that violates the octet rule?
(Multiple Choice)
4.8/5  (46)
(46)
How many lone pairs of electrons are present in the Lewis structure of ammonia, NH3?
(Multiple Choice)
4.8/5  (46)
(46)
Which of the statements concerning chemical bonds is false?
(Multiple Choice)
4.8/5  (34)
(34)
Predict the bond angles around the carbon atom in the structure of carbonic acid shown below. Don't forget to draw in lone pairs where needed to give octets. 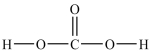
(Multiple Choice)
4.8/5  (35)
(35)
Aspartic acid is an amino acid used to synthesize proteins. There are _____ atoms with a trigonal planar geometry in the aspartic acid structure shown here. 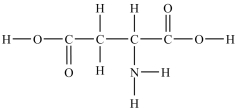
(Short Answer)
4.9/5  (28)
(28)
How many nonbonded electron pairs are in the Lewis structure below? 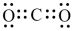
(Multiple Choice)
4.8/5  (41)
(41)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)