Exam 4: Covalent Compounds
Exam 1: Matter and Measurement89 Questions
Exam 2: Atoms and the Periodic Table90 Questions
Exam 3: Ionic Compounds90 Questions
Exam 4: Covalent Compounds90 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: Energy Changes, Reaction Rates, and Equilibrium88 Questions
Exam 7: Gases, Liquids, and Solids79 Questions
Exam 8: Solutions90 Questions
Exam 9: Acids and Bases90 Questions
Exam 10: Nuclear Chemistry85 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups103 Questions
Exam 12: Alkanes106 Questions
Exam 13: Unsaturated Hydrocarbons101 Questions
Exam 14: Organic Compounds That Contain Oxygen, Halogen, or Sulfur111 Questions
Exam 15: The Three-Dimensional Shape of Molecules100 Questions
Exam 16: Aldehydes and Ketones103 Questions
Exam 17: Carboxylic Acids, Esters, and Amides81 Questions
Exam 18: Amines and Neurotransmitters105 Questions
Exam 19: Lipids105 Questions
Exam 20: Carbohydrates92 Questions
Exam 21: Amino Acids, Proteins, and Enzymes88 Questions
Exam 22: Nucleic Acids and Protein Synthesis89 Questions
Exam 23: Digestion and the Conversion of Food Into Energy92 Questions
Exam 24: Carbohydrate, Lipid, and Protein Metabolism91 Questions
Select questions type
Considering the electronegativity values indicated for each element, which covalent bond has the least degree of polarity? 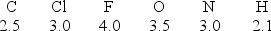
(Multiple Choice)
4.9/5  (35)
(35)
The Lewis structure for BH3 contains an atom that does not follow the octet rule.
(True/False)
4.9/5  (38)
(38)
A molecule that contains only one polar bond is a polar molecule.
(True/False)
4.8/5  (36)
(36)
Atoms with seven valence electrons typically form one covalent bond.
(True/False)
4.8/5  (31)
(31)
C-H bonds are considered to be nonpolar, because the electronegativity difference between carbon and hydrogen is small.
(True/False)
4.8/5  (37)
(37)
To represent the three-dimensional geometry of a tetrahedron on a two-dimensional piece of paper, a solid line is used for the two bonds in the plane of the paper; a _____ is used to show a bond that extends in front of the plane; and a _____ is used to show a bond that extends behind the plane.
(Short Answer)
4.7/5  (30)
(30)
In the Lewis structure of a molecule, oxygen atoms typically have _____ lone pair(s)of electrons.
(Short Answer)
4.9/5  (32)
(32)
Which element may have more than eight valence electrons around it when present in a covalent compound?
(Multiple Choice)
5.0/5  (41)
(41)
A Lewis structure shows the connectivity between atoms in a molecule, as well as where all the bonding and nonbonding valence electrons reside.
(True/False)
4.8/5  (37)
(37)
In general, a _____ bond will be one in which the electronegativity difference between two atoms is 0.5 units or greater.
(Short Answer)
4.8/5  (35)
(35)
How many valence electrons are in a molecule of formaldehyde (CH2O)?
(Multiple Choice)
4.7/5  (40)
(40)
What is the molecular shape around the phosphorus atom in PH3?
(Multiple Choice)
4.9/5  (33)
(33)
Rank the atoms Br, Cl, and F in order of increasing electronegativity.
(Multiple Choice)
4.8/5  (41)
(41)
Showing 41 - 60 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)