Exam 3: Ionic Compounds
Exam 1: Matter and Measurement89 Questions
Exam 2: Atoms and the Periodic Table90 Questions
Exam 3: Ionic Compounds90 Questions
Exam 4: Covalent Compounds90 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: Energy Changes, Reaction Rates, and Equilibrium88 Questions
Exam 7: Gases, Liquids, and Solids79 Questions
Exam 8: Solutions90 Questions
Exam 9: Acids and Bases90 Questions
Exam 10: Nuclear Chemistry85 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups103 Questions
Exam 12: Alkanes106 Questions
Exam 13: Unsaturated Hydrocarbons101 Questions
Exam 14: Organic Compounds That Contain Oxygen, Halogen, or Sulfur111 Questions
Exam 15: The Three-Dimensional Shape of Molecules100 Questions
Exam 16: Aldehydes and Ketones103 Questions
Exam 17: Carboxylic Acids, Esters, and Amides81 Questions
Exam 18: Amines and Neurotransmitters105 Questions
Exam 19: Lipids105 Questions
Exam 20: Carbohydrates92 Questions
Exam 21: Amino Acids, Proteins, and Enzymes88 Questions
Exam 22: Nucleic Acids and Protein Synthesis89 Questions
Exam 23: Digestion and the Conversion of Food Into Energy92 Questions
Exam 24: Carbohydrate, Lipid, and Protein Metabolism91 Questions
Select questions type
Ionic compounds are composed of positively and negatively charged ions held together by strong electrostatic forces.
(True/False)
4.9/5  (37)
(37)
Anions are formed when a neutral atom gains one or more electrons.
(True/False)
4.9/5  (42)
(42)
Ionic bonds form between a metal on the _____ side of the periodic table and a nonmetal on the _____ side.
(Short Answer)
4.7/5  (37)
(37)
The diphosphate ion is a biologically important polyatomic ion. If the ionic compound calcium diphosphate has the formula Ca2P2O7, which correctly represents the ion symbol of the diphosphate ion?
(Multiple Choice)
4.9/5  (37)
(37)
The noble gas that has the same electronic configuration as the iodide ion is krypton.
(True/False)
4.9/5  (44)
(44)
In crystal of an ionic compound, the cations are surrounded by anions so that the charges of the ions are balanced.
(True/False)
4.8/5  (37)
(37)
Sodium bicarbonate and sodium hydrogen carbonate are two acceptable names for the same compound.
(True/False)
4.8/5  (38)
(38)
An ionic compound is a pure substance formed by chemically combining two or more nonmetal atoms together.
(True/False)
4.7/5  (28)
(28)
Which statement best explains the basis for the octet rule?
(Multiple Choice)
5.0/5  (32)
(32)
When forming an ion, an atom with the electron-dot symbol of 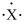 forms an anion with a -3 charge by the loss of three electrons.
forms an anion with a -3 charge by the loss of three electrons.
(True/False)
4.7/5  (36)
(36)
Showing 61 - 80 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)