Exam 1: Matter and Measurement
Exam 1: Matter and Measurement89 Questions
Exam 2: Atoms and the Periodic Table90 Questions
Exam 3: Ionic Compounds90 Questions
Exam 4: Covalent Compounds90 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: Energy Changes, Reaction Rates, and Equilibrium88 Questions
Exam 7: Gases, Liquids, and Solids79 Questions
Exam 8: Solutions90 Questions
Exam 9: Acids and Bases90 Questions
Exam 10: Nuclear Chemistry85 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups103 Questions
Exam 12: Alkanes106 Questions
Exam 13: Unsaturated Hydrocarbons101 Questions
Exam 14: Organic Compounds That Contain Oxygen, Halogen, or Sulfur111 Questions
Exam 15: The Three-Dimensional Shape of Molecules100 Questions
Exam 16: Aldehydes and Ketones103 Questions
Exam 17: Carboxylic Acids, Esters, and Amides81 Questions
Exam 18: Amines and Neurotransmitters105 Questions
Exam 19: Lipids105 Questions
Exam 20: Carbohydrates92 Questions
Exam 21: Amino Acids, Proteins, and Enzymes88 Questions
Exam 22: Nucleic Acids and Protein Synthesis89 Questions
Exam 23: Digestion and the Conversion of Food Into Energy92 Questions
Exam 24: Carbohydrate, Lipid, and Protein Metabolism91 Questions
Select questions type
One-thousand (1, 000)ms is the same length of time as one (1) s.
(True/False)
4.9/5  (40)
(40)
The estimated average daily requirement of folic acid for pregnant females is 520 micrograms. Which accurately expresses this value?
(Multiple Choice)
4.8/5  (38)
(38)
A beaker contains 145.675 mL of a saline solution. If 24.2 mL of the saline solution are removed from the beaker, what volume of solution remains?
(Multiple Choice)
4.8/5  (35)
(35)
The density of human urine is normally between 1.003 and 1.030g/mL, and is often used as a diagnostic tool. If a 25.00 mL sample of urine from a patient has a mass of 26.875 g, how does the density of the urine sample compare to the normal range?
(Multiple Choice)
4.8/5  (41)
(41)
The two conversion factors for the equality 1 in = 2.54 cm are properly shown below. 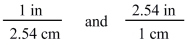
(True/False)
4.9/5  (42)
(42)
What is the mass in grams of 85.32 mL of blood plasma with a density of 1.03 g/mL?
(Multiple Choice)
4.9/5  (39)
(39)
What is the mass in kilograms of an individual who weighs 197 lb?
(Multiple Choice)
4.9/5  (38)
(38)
A mixture can be separated into its components by physical changes.
(True/False)
4.7/5  (42)
(42)
If a 185-lb patient is prescribed 145 mg of the cholesterol lowering drug Tricor daily, what dosage is the patient receiving in mg/kg of his body weight?
(Multiple Choice)
4.9/5  (31)
(31)
Which volume has the most uncertainty associated with the measurement?
(Multiple Choice)
4.8/5  (38)
(38)
When the liquid carbon tetrachloride (density = 1.59 g/mL)is added to water, the top layer will be the water layer.
(True/False)
4.8/5  (33)
(33)
If a piece of rock has a volume of 0.73 L and a mass of 1524 g, what is the density of the rock in g/mL?
(Multiple Choice)
4.9/5  (38)
(38)
Specific gravity is a quantity that compares the density of a substance with the density of water.
(True/False)
4.7/5  (39)
(39)
When the measurement 340, 942 s is rounded to two significant figures, the value is properly reported as _____.
(Short Answer)
4.9/5  (43)
(43)
Showing 41 - 60 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)