Exam 19: Transition Metals and Coordination Chemistry
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
Analysis of the data from a titration indicates that a 0.1000-g sample of the complex contains 0.0708 g of py. Further analysis shows that 0.1000 g of the complex contains 0.0132 g of cobalt and 0.0160 g of chloride. What is the empirical formula of the complex?
(Multiple Choice)
4.9/5  (42)
(42)
Addition of AgNO3 to aqueous solutions of the complex results in a cloudy white precipitate, presumably AgCl. You dissolve 0.1000 g of the complex in H2O and perform a precipitation titration with 0.0500 M AgNO3 as the titrant. Using an electrode that is sensitive to [Ag+], you reach the endpoint after 9.00 mL of titrant is added. How many grams of chloride ion were present in the 0.1000-g sample?
(Multiple Choice)
4.9/5  (33)
(33)
The complex ions of Zn2+ are all colorless. The most likely explanation for this is that
(Multiple Choice)
4.8/5  (41)
(41)
For which of the following metal ions would there be no distinction between low spin and high spin in octahedral complexes?
(Multiple Choice)
4.7/5  (39)
(39)
Explain the toxicities of carbon monoxide (CO) and the cyanide ion (CN-).
(Short Answer)
4.8/5  (33)
(33)
Consider the pseudo-octahedral complex of Cr3+ shown below, where A and B represent Lewis bases and where A produces a stronger crystal field than B. Draw an appropriate crystal field diagram for this complex (include the electrons). 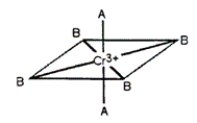
(Short Answer)
4.8/5  (35)
(35)
The 3d electrons in Co(NH3)63+ are all paired, but Fe(H2O)62+ has unpaired electrons (is paramagnetic). Explain.
(Essay)
4.8/5  (42)
(42)
How many unpaired electrons are found in [CoCl6]3- (weak field)?
(Multiple Choice)
4.9/5  (39)
(39)
Which transition metal can exist in all oxidation states from +2 to +7?
(Multiple Choice)
4.7/5  (43)
(43)
The complex ion Co(NH3)62+ (three unpaired electrons) is classified as
(Multiple Choice)
4.9/5  (37)
(37)
Give the number of geometric isomers for the octahedral compound [Ma2B2C2], where A, B, and C represent ligands.
(Multiple Choice)
4.8/5  (36)
(36)
Oxygen is stored in mammalian tissue in which type of molecule?
(Multiple Choice)
4.8/5  (37)
(37)
In which of the following complexes does the transition metal have a d8 configuration?
(Multiple Choice)
4.9/5  (40)
(40)
The empirical formula of a compound with a mass percent composition of 6.78% H, 31.43% N, 39.76% Cl, and 22.03% Co is consistent with which of the following complexes?
(Multiple Choice)
4.8/5  (43)
(43)
Fluoride ion ranks low in the spectrochemical series and produces a weak crystal field in complex ions. Based on this information, predict the number of unpaired electrons in [CoF6]3-.
(Multiple Choice)
4.9/5  (32)
(32)
Specify the number of unpaired electrons in Co(en) 33+ (strong field).
(Multiple Choice)
4.9/5  (31)
(31)
Showing 81 - 96 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)