Exam 16: Liquids and Solids
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
Which one of the following statements about solid Cu (face-centered cubic unit cell) is incorrect?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
A
Properties of liquids lie (closer to/further from) properties of a solid than to (or from) properties of a gas.
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
A
Which of the following is the correct order of boiling points for NaNO3, CH3OH, C3H8, and He?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
B
Shown below is a phase diagram for compound X. At 25°C and 1 atm, in what state will X exist? 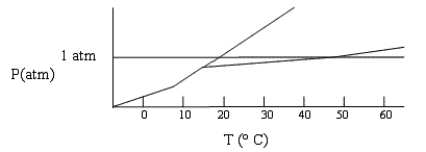
(Multiple Choice)
4.8/5  (33)
(33)
In which of the following processes is energy evolved as heat?
(Multiple Choice)
4.7/5  (44)
(44)
If equal, rigid spheres are arranged in a simple cubic lattice in the usual way (that is, in such a way that they touch each other), what fraction of the corresponding solid will be empty space? [The volume of a sphere is (4/3)πr3, with π = 3.14.]
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following statements is(are) false?
I.The hexagonal closest-packed structure is ABAB....
II.A body-centered cubic unit cell has four atoms per unit cell.
III.For unit cells having the same edge length, a simple cubic structure would have a smaller density than a body-centered cube.
IV.Atoms in a solid consisting of only one element would have six nearest neighbors if the crystal structure was a simple cubic array.
(Multiple Choice)
4.8/5  (48)
(48)
A certain compound with a molar mass of 120.0 g/mol crystallizes with the sodium chloride (rock salt) structure. The length of an edge of the unit cell is 461 pm. What is the density of this compound?
(Multiple Choice)
4.9/5  (28)
(28)
Which is generally larger, the heat of fusion or the heat of vaporization for a given substance? Explain your answer.
(Short Answer)
4.8/5  (35)
(35)
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. A large increase in pressure will
(Multiple Choice)
4.9/5  (34)
(34)
Below is a phase diagram for compound X. You wish to purify a sample of X that was collected at P = 1.0 atm and T = 100 by subliming it. In order to sublime the sample, you should 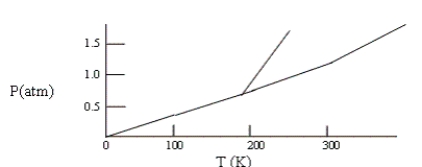
(Multiple Choice)
4.7/5  (30)
(30)
What is the net number of face-centered atoms contained in a face-centered cubic unit cell?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following chemical species has the highest boiling point?
(Multiple Choice)
4.9/5  (35)
(35)
The triple point of CO2 is at 5.2 atm and -57°C. Under atmospheric conditions present in a typical Boulder, Colorado, laboratory (P = 630 torr, T = 23° C), solid CO2 will
(Multiple Choice)
4.7/5  (37)
(37)
The molar volume of a certain form of solid lead is 18 cm3/mol. Assuming cubic closest-packed structure, determine the following.
-The volume of a single cell
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following statements is/are true of carbon nanotubes?
1. Carbon nanotubes contain networks of carbon atoms in interconnected eight-membered rings organized into slender tubes.
2. Carbon nanotubes are lighter and stronger than steel wire.
3. Carbon nanotubes are better electrical conductors than copper wire.
(Multiple Choice)
4.7/5  (30)
(30)
Showing 1 - 20 of 111
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)