Exam 40: All About Atoms
Exam 1: Measurement37 Questions
Exam 2: Motion Along a Straight Line90 Questions
Exam 3: Vector37 Questions
Exam 4: Motion in Two and Three Dimensions56 Questions
Exam 5: Force and Motion I73 Questions
Exam 6: Force and Motion II74 Questions
Exam 7: Kinetic Energy and Work73 Questions
Exam 8: Potential Energy and Conservation of Energy63 Questions
Exam 9: Center of Mass and Linear Momentum99 Questions
Exam 10: Rotation102 Questions
Exam 11: Rolling, Torque, and Angular Momentum66 Questions
Exam 12: Equilibrium and Elasticity57 Questions
Exam 13: Gravitation55 Questions
Exam 14: Fluids88 Questions
Exam 15: Oscillations75 Questions
Exam 16: Waves I82 Questions
Exam 17: Waves II71 Questions
Exam 18: Temperature, Heat, and the First Law of Thermodynamics96 Questions
Exam 19: The Kinetic Theory of Gases113 Questions
Exam 20: Entropy and the Second Law of Thermodynamics61 Questions
Exam 21: Electric Charge52 Questions
Exam 22: Electric Fields55 Questions
Exam 23: Gauss Law38 Questions
Exam 24: Electric Potential52 Questions
Exam 25: Capacitance61 Questions
Exam 26: Current and Resistance55 Questions
Exam 27: Circuits73 Questions
Exam 28: Magnetic Fields55 Questions
Exam 29: Magnetic Fields Due to Currents49 Questions
Exam 30: Induction and Inductance90 Questions
Exam 31: Electromagnetic Oscillations and Alternating Current88 Questions
Exam 32: Maxwells Equations; Magnetism of Matter81 Questions
Exam 33: Electromagnetic Waves83 Questions
Exam 34: Images79 Questions
Exam 35: Interference46 Questions
Exam 36: Diffraction77 Questions
Exam 37: Relativity68 Questions
Exam 38: Photons and Matter Waves57 Questions
Exam 39: More About Matter Waves41 Questions
Exam 40: All About Atoms79 Questions
Exam 41: Conduction of Electricity in Solids51 Questions
Exam 42: Nuclear Physics68 Questions
Exam 43: Energy From the Nucleus50 Questions
Exam 44: Quarks, Leptons, and the Big Bang55 Questions
Select questions type
The force exerted on a magnetic dipole as it moves with velocity through a Stern-Gerlach apparatus is:
(Multiple Choice)
4.9/5  (24)
(24)
Which of the following is essential for laser action to occur between two energy levels of an atom?
(Multiple Choice)
4.8/5  (26)
(26)
An electron in an atom is in a state with ℓ=3 and mℓ =2. The angle between and the z axis is:
(Multiple Choice)
4.9/5  (36)
(36)
The transition shown gives rise to an x-ray. The correct label for this is: 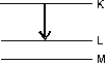
(Multiple Choice)
4.7/5  (43)
(43)
Electrons are in a two-dimensional square potential energy well with sides of length L. The potential energy is infinite at the sides and zero inside. The single-particle energies are given by where nx and ny are integers. The number of single-particle states with energy 5(h2/8mL2) is:
(Multiple Choice)
4.8/5  (27)
(27)
The K x rays arising from a cobalt (Z = 27) target have a wavelength of about 179 pm. The atomic number of a target that gives rise to K x rays with a wavelength one-third as great ( 60pm) is:
(Multiple Choice)
4.9/5  (27)
(27)
An electron is in a quantum state for which there are seven allowed values of the z component of the angular momentum. The magnitude of the angular momentum is:
(Multiple Choice)
4.8/5  (39)
(39)
The states being filled from the beginning to end of the lanthanide series of atoms are:
(Multiple Choice)
4.9/5  (33)
(33)
The magnitude of the spin magnetic dipole moment of an atom is ( B is the Bohr magneton, and s is a positive number):
(Multiple Choice)
4.9/5  (31)
(31)
The most energetic electron in any atom at the end of a period of the periodic table is in:
(Multiple Choice)
4.8/5  (35)
(35)
The most energetic electron in any atom at the beginning of a period of the periodic table is in:
(Multiple Choice)
4.9/5  (28)
(28)
The number of values of the orbital quantum number ℓ associated with the principal quantum number n = 3 is:
(Multiple Choice)
4.8/5  (32)
(32)
Six electrons are in a two-dimensional square potential energy well with sides of length L. The potential energy is infinite at the sides and zero inside. The single-particle energies are given by where nx and ny are integers. If a seventh electron is added to the system when it is in its ground state the least energy the additional electron can have is:
(Multiple Choice)
4.7/5  (35)
(35)
The magnetic quantum number mℓ is most closely associated with what property of the electron in an atom?
(Multiple Choice)
4.9/5  (38)
(38)
An electron in an atom is in a state with ℓ=5. The minimum angle between and the z axis is:
(Multiple Choice)
4.9/5  (32)
(32)
The group of atoms at the beginning of periods of the periodic table is called:
(Multiple Choice)
4.8/5  (42)
(42)
Showing 61 - 79 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)