Exam 18: Temperature, Heat, and the First Law of Thermodynamics
Exam 1: Measurement37 Questions
Exam 2: Motion Along a Straight Line90 Questions
Exam 3: Vector37 Questions
Exam 4: Motion in Two and Three Dimensions56 Questions
Exam 5: Force and Motion I73 Questions
Exam 6: Force and Motion II74 Questions
Exam 7: Kinetic Energy and Work73 Questions
Exam 8: Potential Energy and Conservation of Energy63 Questions
Exam 9: Center of Mass and Linear Momentum99 Questions
Exam 10: Rotation102 Questions
Exam 11: Rolling, Torque, and Angular Momentum66 Questions
Exam 12: Equilibrium and Elasticity57 Questions
Exam 13: Gravitation55 Questions
Exam 14: Fluids88 Questions
Exam 15: Oscillations75 Questions
Exam 16: Waves I82 Questions
Exam 17: Waves II71 Questions
Exam 18: Temperature, Heat, and the First Law of Thermodynamics96 Questions
Exam 19: The Kinetic Theory of Gases113 Questions
Exam 20: Entropy and the Second Law of Thermodynamics61 Questions
Exam 21: Electric Charge52 Questions
Exam 22: Electric Fields55 Questions
Exam 23: Gauss Law38 Questions
Exam 24: Electric Potential52 Questions
Exam 25: Capacitance61 Questions
Exam 26: Current and Resistance55 Questions
Exam 27: Circuits73 Questions
Exam 28: Magnetic Fields55 Questions
Exam 29: Magnetic Fields Due to Currents49 Questions
Exam 30: Induction and Inductance90 Questions
Exam 31: Electromagnetic Oscillations and Alternating Current88 Questions
Exam 32: Maxwells Equations; Magnetism of Matter81 Questions
Exam 33: Electromagnetic Waves83 Questions
Exam 34: Images79 Questions
Exam 35: Interference46 Questions
Exam 36: Diffraction77 Questions
Exam 37: Relativity68 Questions
Exam 38: Photons and Matter Waves57 Questions
Exam 39: More About Matter Waves41 Questions
Exam 40: All About Atoms79 Questions
Exam 41: Conduction of Electricity in Solids51 Questions
Exam 42: Nuclear Physics68 Questions
Exam 43: Energy From the Nucleus50 Questions
Exam 44: Quarks, Leptons, and the Big Bang55 Questions
Select questions type
The thermal energy of an object is associated with:
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
D
The two metallic strips that constitute some thermostats must differ in:
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
E
To help keep buildings cool in the summer, dark colored window shades have been replaced by light colored shades. This is because light colored shades:
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
C
A homeowner purchases insulation for her attic rated at R-15. She wants the attic insulated to R-30. If the insulation she purchased is 10 cm thick, what thickness does she need to use?
(Multiple Choice)
4.8/5  (37)
(37)
In the first law of thermodynamics, the change in internal energy ΔEint:
(Multiple Choice)
4.8/5  (38)
(38)
The figure shows a rectangular brass plate at 0 C in which there is cut a rectangular hole of dimensions indicated. If the temperature of the plate is raised to 150 C: 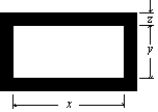
(Multiple Choice)
4.8/5  (34)
(34)
Ten grams of ice at -20 C is to be changed to steam at 130 C. The specific heat of both ice and steam is 0.5 cal/g. C. The specific heat of water is 1.00 cal/g .K. The heat of fusion is 80 cal/g and the heat of vaporization is 540 cal/g. The entire process requires:
(Multiple Choice)
4.9/5  (36)
(36)
The coefficient of expansion of a certain type of steel is 0.000012 per C . The coefficient of volume expansion is:
(Multiple Choice)
4.9/5  (31)
(31)
Two different samples have the same mass and temperature. Equal quantities of energy are absorbed as heat by each. Their final temperatures may be different because the samples have different:
(Multiple Choice)
4.9/5  (31)
(31)
When the temperature of a copper penny is increased by 100 C , its diameter increases by 0.17%. The area of one of its faces increases by:
(Multiple Choice)
4.9/5  (32)
(32)
The diagram shows four thermometers, labeled W, X, Y, and Z. The freezing and boiling points of water are indicated. Rank the thermometers according to the size of a degree on their scales, smallest to largest. 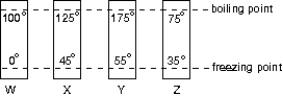
(Multiple Choice)
4.9/5  (33)
(33)
In constructing a thermometer it is NECESSARY to use a substance that:
(Multiple Choice)
5.0/5  (31)
(31)
The heat capacity of object B is twice that of object A. Initially A is at 300 K and B is at 450 K. They are placed in thermal contact and the combination is isolated. The final temperature of both objects is:
(Multiple Choice)
4.9/5  (40)
(40)
Constant-volume gas thermometers using different gases all indicate nearly the same temperature when in contact with the same object if:
(Multiple Choice)
4.7/5  (36)
(36)
Of the following which might NOT be zero over one cycle of a cyclic process?
(Multiple Choice)
4.7/5  (48)
(48)
Take the mechanical equivalent of heat as 4 J/cal. A 10-gram bullet moving at 2000 m/s plunges into 1 kg of paraffin wax (specific heat 0.7 cal/g . C). The wax was initially at 20 C. Assuming that all the bullet's energy heats the wax, its final temperature is:
(Multiple Choice)
4.8/5  (32)
(32)
Showing 1 - 20 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)