Exam 20: Entropy and the Second Law of Thermodynamics
Exam 1: Measurement37 Questions
Exam 2: Motion Along a Straight Line90 Questions
Exam 3: Vectors43 Questions
Exam 4: Motion in Two and Three Dimensions56 Questions
Exam 5: Force and Motion73 Questions
Exam 6: Force and Motion74 Questions
Exam 7: Kinetic Energy and Work73 Questions
Exam 8: Potential Energy and Conservation of Energy65 Questions
Exam 9: Center of Mass and Linear Momentum99 Questions
Exam 10: Rotation102 Questions
Exam 11: Rolling, Torque, and Angular Momentum67 Questions
Exam 12: Equilibrium and Elasticity57 Questions
Exam 13: Gravitation61 Questions
Exam 14: Fluids91 Questions
Exam 15: Oscillations80 Questions
Exam 16: Waves83 Questions
Exam 17: Waves72 Questions
Exam 18: Temperature, Heat, and the First Law of Thermodynamics96 Questions
Exam 19: The Kinetic Theory of Gases114 Questions
Exam 20: Entropy and the Second Law of Thermodynamics61 Questions
Exam 21: Coulombs Law52 Questions
Exam 22: Electric Fields55 Questions
Exam 23: Gauss Law44 Questions
Exam 24: Electric Potential55 Questions
Exam 25: Capacitance61 Questions
Exam 26: Current and Resistance55 Questions
Exam 27: Circuits75 Questions
Exam 28: Magnetic Fields53 Questions
Exam 29: Magnetic Fields Due to Currents49 Questions
Exam 30: Induction and Inductance90 Questions
Exam 31: Electromagnetic Oscillations and Alternating Current89 Questions
Exam 32: Maxwells Equations; Magnetism of Matter87 Questions
Exam 33: Electromagnetic Waves83 Questions
Exam 34: Images79 Questions
Exam 35: Interference 147 Questions
Exam 36: Diffraction77 Questions
Exam 37: Relativity69 Questions
Exam 38: Photons and Matter Waves59 Questions
Exam 39: More About Matter Waves45 Questions
Exam 40: All About Atoms79 Questions
Exam 41: Conduction of Electricity in Solids51 Questions
Exam 42: Energy From the Nucleus50 Questions
Exam 43: Quarks, Leptons, and the Big Bang59 Questions
Select questions type
A Carnot engine operates between 200°C and 20°C.Its maximum possible efficiency is:
(Multiple Choice)
4.9/5  (34)
(34)
The temperature of n moles of a gas is increased from Ti to Tf at constant pressure.If the molar specific heat at constant pressure is Cp and is independent of temperature, then change in the entropy of the gas is:
(Multiple Choice)
4.7/5  (39)
(39)
A Carnot heat engine and an irreversible heat engine both operate between the same high temperature and low temperature reservoirs.They absorb the same heat from the high temperature reservoir as heat.The irreversible engine:
(Multiple Choice)
4.7/5  (35)
(35)
Is it possible to transfer energy from a low-temperature reservoir to a high-temperature reservoir?
(Multiple Choice)
4.8/5  (35)
(35)
A heat engine absorbs energy of magnitude 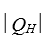 from a high temperature reservoir, does work of magnitude
from a high temperature reservoir, does work of magnitude 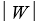 , and transfers energy of magnitude
, and transfers energy of magnitude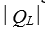 as heat to a low temperature reservoir.Its efficiency is:
as heat to a low temperature reservoir.Its efficiency is:
(Multiple Choice)
4.9/5  (38)
(38)
Consider all possible isothermal contractions of an ideal gas.The entropy of the gas:
(Multiple Choice)
4.8/5  (33)
(33)
A heat engine operates between a high temperature reservoir at TH and a low temperature reservoir at TL.Its efficiency is given by 1 - TL/TH:
(Multiple Choice)
4.9/5  (37)
(37)
Twenty-five identical molecules are in a box.Microstates are designated by identifying the molecules in the left and right halves of the box.The Boltzmann constant is 1.38 *10-23 J/K.The entropy associated with the configuration for which 15 molecules are in the left half and 10 molecules are in the right half is:
(Multiple Choice)
4.8/5  (36)
(36)
Rank from smallest to largest, the changes in entropy of a pan of water on a hot plate, as the temperature of the water 
(Multiple Choice)
4.8/5  (44)
(44)
Consider the following processes:  Which are never found to occur?
Which are never found to occur?
(Multiple Choice)
4.8/5  (36)
(36)
Let k be the Boltzmann constant.If the configuration of molecules in a gas changes from one with a multiplicity of M1 to one with a multiplicity of M2, then entropy changes by:
(Multiple Choice)
4.9/5  (41)
(41)
Twenty-five identical molecules are in a box.Microstates are designated by identifying the molecules in the left and right halves of the box.The multiplicity of the configuration with 15 molecules in the right half and 10 molecules in the left half is:
(Multiple Choice)
4.9/5  (30)
(30)
An ideal gas, consisting of n moles, undergoes an irreversible process in which the temperature has the same value at the beginning and end.If the volume changes from Vi to Vf, the change in entropy is given by:
(Multiple Choice)
4.9/5  (37)
(37)
A reversible refrigerator operates between a low temperature reservoir at TL and a high temperature reservoir at TH.Its coefficient of performance is given by:
(Multiple Choice)
4.9/5  (35)
(35)
Let k be the Boltzmann constant.If the configuration of the molecules in a gas changes so that the multiplicity is reduced to one-third its previous value, the entropy of the gas changes by:
(Multiple Choice)
5.0/5  (41)
(41)
Showing 21 - 40 of 61
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)