Exam 1: The Foundations of Biochemistry
Exam 1: The Foundations of Biochemistry109 Questions
Exam 2: Water87 Questions
Exam 3: Amino Acids, Peptides, and Proteins112 Questions
Exam 4: The Three-Dimensional Structure of Proteins100 Questions
Exam 5: Protein Function101 Questions
Exam 6: Enzymes106 Questions
Exam 7: Carbohydrates and Glycobiology109 Questions
Exam 8: Nucleotides and Nucleic Acids98 Questions
Exam 9: DNA-Based Information Technologies102 Questions
Exam 10: Lipids102 Questions
Exam 11: Biological Membranes and Transport105 Questions
Exam 12: Biosignaling98 Questions
Exam 13: Bioenergetics and Biochemical Reaction Types105 Questions
Exam 14: Glycolysis, Gluconeogenesis, and the Pentose Phosphate Pathway112 Questions
Exam 15: Principles of Metabolic Regulation101 Questions
Exam 16: The Citric Acid Cycle105 Questions
Exam 17: Fatty Acid Catabolism97 Questions
Exam 18: Amino Acid Oxidation and the Production of Urea98 Questions
Exam 19: Oxidative Phosphorylation and Photophosphorylation96 Questions
Exam 20: Carbohydrate Biosynthesis in Plants and Bacteria94 Questions
Exam 21: Lipid Biosynthesis100 Questions
Exam 22: Biosynthesis of Amino Acids, Nucleotides, and Related Molecules98 Questions
Exam 23: Hormonal Regulation and Integration of Mammalian Metabolism100 Questions
Exam 24: Genes and Chromosomes99 Questions
Exam 25: DNA Metabolism101 Questions
Exam 26: RNA Metabolism101 Questions
Exam 27: Protein Metabolism99 Questions
Exam 28: Regulation of Gene Expression99 Questions
Select questions type
Why is the use of the expression "Mr = 18,000 daltons" incorrect?
(Short Answer)
4.8/5  (35)
(35)
The three-dimensional structure of macromolecules is formed and maintained primarily through noncovalent interactions. Which one of the following is NOT considered a noncovalent interaction?
(Multiple Choice)
4.8/5  (34)
(34)
Describe the relationship between a living organism and its surroundings in terms of both matter and energy.
(Essay)
4.9/5  (47)
(47)
Discuss how a mutation in DNA could be harmful or beneficial to an organism.
(Essay)
4.9/5  (38)
(38)
Reaction 1 has a G° of -12.3 kJ/mol, and Reaction 2 has a G° of 23.4 kJ/mol. Which statement is TRUE of these two reactions?
(Multiple Choice)
4.7/5  (26)
(26)
The joining of two amino acids via a peptide bond (the process of protein synthesis) has a positive G value. What does this imply?
(Multiple Choice)
4.7/5  (45)
(45)
In an oxidation-reduction reaction, the oxidized reagent _____, and the reduced reagent _____.
(Multiple Choice)
4.8/5  (37)
(37)
What is the CORRECT name for the configuration of the molecule shown in the figure? 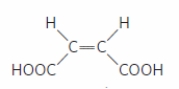
(Multiple Choice)
4.9/5  (38)
(38)
_____ are typically expressed under all conditions and are not subject to regulation.
(Multiple Choice)
4.9/5  (38)
(38)
List the types of noncovalent interactions that are important in providing stability to the three-dimensional structures of macromolecules. (b) Why is it important that these interactions be noncovalent, rather than covalent, bonds?
(Short Answer)
4.8/5  (34)
(34)
What six characteristics distinguish living organisms from inanimate objects?
(Short Answer)
4.9/5  (45)
(45)
Humans maintain a nearly constant level of hemoglobin by continually synthesizing and degrading it. This is an example of a(n):
(Multiple Choice)
5.0/5  (38)
(38)
All cells are surrounded by a plasma membrane composed of lipid and protein molecules. What is the function of the plasma membrane?
(Short Answer)
4.9/5  (28)
(28)
Which group of single-celled microorganisms has many members found growing in extreme environments?
(Multiple Choice)
4.7/5  (37)
(37)
Showing 81 - 100 of 109
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)