Exam 11: Liquids and Solids
Exam 1: Introduction to Chemistry49 Questions
Exam 2: The Metric System183 Questions
Exam 3: Matter and Energy186 Questions
Exam 4: Models of the Atom173 Questions
Exam 5: The Periodic Table209 Questions
Exam 6: Language of Chemistry194 Questions
Exam 7: Chemical Reactions169 Questions
Exam 8: The Mole Concept131 Questions
Exam 9: Chemical Equation Calculations152 Questions
Exam 10: Gases156 Questions
Exam 11: Liquids and Solids180 Questions
Exam 12: Chemical Bonding200 Questions
Exam 13: Solutions120 Questions
Exam 14: Acids and Bases155 Questions
Exam 15: Advanced Problem Solving111 Questions
Exam 16: Chemical Equilibrium135 Questions
Exam 17: Oxidation and Reduction144 Questions
Exam 18: Nuclear Chemistry150 Questions
Exam 19: Organic Chemistry191 Questions
Exam 20: Biochemistry144 Questions
Exam 21: Interlude: Prerequisite Science Skills103 Questions
Select questions type
An unknown hydrate of iron(II)sulfate,FeSO₄ • XH₂O,is heated to give 45.3% water.What is the water of crystallization (X)for the hydrate?
(Multiple Choice)
4.9/5  (32)
(32)
Consider the following liquids with similar molar masses.Predict which liquid has the weakest intermolecular attraction based on surface tension data.
(Multiple Choice)
5.0/5  (34)
(34)
Calculate the heat necessary to convert 10.0 g of solid benzene at its melting point to vapor at its boiling point.The specific heat of liquid benzene is 0.580 cal/(g x °C),the heat of fusion for benzene is 31.0 cal/g,and the heat of vaporization is 94.0 cal/g.The melting point of benzene is 5.5 °C and the boiling point is 80.1 °C.
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following is true of the intermolecular attractions in liquids?
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following describes a molecular liquid conceptually?
(Multiple Choice)
4.8/5  (36)
(36)
Calculate the heat required to convert 10.0 g of ice at 0.0 °C to steam at 100.0 °C.The specific heat of water is 1.00 cal/(g x °C);the heat of fusion is 80.0 cal/g;and the heat of vaporization is 540.0 cal/g.
(Multiple Choice)
4.9/5  (38)
(38)
Calculate the heat required to convert 10.0 g of water at 25.0 °C to steam at 100.0 °C.The specific heat of water is 1.00 cal/(g x °C);the heat of fusion is 80.0 cal/g;and the heat of vaporization is 540.0 cal/g.
(Multiple Choice)
4.9/5  (36)
(36)
What is the term for a crystalline solid composed of metal atoms which repeat in a regular pattern?
(Multiple Choice)
4.8/5  (37)
(37)
What is the term for the resistance of a liquid to spread and its tendency to form spherical droplets?
(Multiple Choice)
4.8/5  (29)
(29)
What is the difference between bottled water and tap water?
(Multiple Choice)
4.9/5  (36)
(36)
What are the products from the decomposition of a hydrate?
(Multiple Choice)
4.7/5  (42)
(42)
What is the term that describes a compound which has lost water of hydration?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following illustrates the bond polarity between H-O in water?
(Multiple Choice)
4.7/5  (36)
(36)
How many pairs of bonding electrons are in a water molecule?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following accounts for the unusually high heat of fusion for ice?
(Multiple Choice)
4.9/5  (27)
(27)
Predict the physical state of krypton at -175 °C (Mp = -157 °C,Bp = -152 °C)and normal atmospheric pressure.
(Multiple Choice)
4.8/5  (43)
(43)
Complete the following chemical reaction and indicate the products. CH₄(g)+ O₂(g) 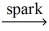
(Multiple Choice)
4.7/5  (39)
(39)
Calculate the heat absorbed when 10.0 g of ice at 0 °C melts to water at the same temperature.The specific heat of water is 1.00 cal/(g x °C);the heat of fusion is 80.0cal/g;and the heat of vaporization is 540.0 cal/g.
(Multiple Choice)
4.7/5  (30)
(30)
Showing 121 - 140 of 180
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)