Exam 11: Liquids and Solids
Exam 1: Introduction to Chemistry49 Questions
Exam 2: The Metric System183 Questions
Exam 3: Matter and Energy186 Questions
Exam 4: Models of the Atom173 Questions
Exam 5: The Periodic Table209 Questions
Exam 6: Language of Chemistry194 Questions
Exam 7: Chemical Reactions169 Questions
Exam 8: The Mole Concept131 Questions
Exam 9: Chemical Equation Calculations152 Questions
Exam 10: Gases156 Questions
Exam 11: Liquids and Solids180 Questions
Exam 12: Chemical Bonding200 Questions
Exam 13: Solutions120 Questions
Exam 14: Acids and Bases155 Questions
Exam 15: Advanced Problem Solving111 Questions
Exam 16: Chemical Equilibrium135 Questions
Exam 17: Oxidation and Reduction144 Questions
Exam 18: Nuclear Chemistry150 Questions
Exam 19: Organic Chemistry191 Questions
Exam 20: Biochemistry144 Questions
Exam 21: Interlude: Prerequisite Science Skills103 Questions
Select questions type
Predict the physical state of argon at -197 °C (Mp = -189 °C,Bp = -186 °C)and normal atmospheric pressure.
(Multiple Choice)
4.8/5  (39)
(39)
An unknown hydrate of sodium dichromate,Na₂Cr₂O₇ • XH₂O,is heated to give 12.1% water.What is the water of crystallization (X)for the hydrate?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following types of crystalline solids is malleable,ductile,and a conductor of electricity?
(Multiple Choice)
4.9/5  (35)
(35)
Consider the following liquids with similar molar masses.Predict which has the weakest intermolecular attraction based only on boiling point data.
(Multiple Choice)
4.8/5  (34)
(34)
Consider the following liquids with similar molar masses.Predict which has the strongest intermolecular attraction based only on boiling point data.
(Multiple Choice)
4.9/5  (34)
(34)
What is the term for the temperature at which the vapor pressure of a liquid is equal to the atmospheric pressure?
(Multiple Choice)
4.8/5  (41)
(41)
Complete the following chemical reaction and indicate the products. C₂H₄(g)+ O₂(g) 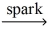
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the heat released when 25.0 g of water at 0 °C crystallizes to ice at the same temperature.The specific heat of water is 1.00 cal/(g x °C);the heat of fusion is 80.0 cal/g;and the heat of vaporization is 540.0 cal/g.
(Multiple Choice)
4.9/5  (35)
(35)
An unknown hydrate of chromium(III)acetate,Cr(C₂H₃O₂)3 • XH₂O,is heated to give 7.29% water.What is the water of crystallization (X)for the hydrate?
(Multiple Choice)
4.8/5  (38)
(38)
What is the chemical formula for strontium nitrate hexahydrate?
(Multiple Choice)
5.0/5  (32)
(32)
If the molecules in a liquid have a strong attraction for each other,which of the following properties has a relatively low value?
(Multiple Choice)
4.8/5  (36)
(36)
Complete the following chemical reaction and indicate the products. HNO₃(aq)+ Ca(OH)2(aq)→
(Multiple Choice)
4.9/5  (42)
(42)
What is the approximate percentage of all Americans who drink bottled water?
(Multiple Choice)
4.8/5  (38)
(38)
Consider the following liquids with similar molar masses.Predict which liquid has the strongest intermolecular attraction based on viscosity data.
(Multiple Choice)
4.9/5  (35)
(35)
What is the term for water containing a variety of different cations and anions?
(Multiple Choice)
4.8/5  (40)
(40)
What is the strongest intermolecular force in a liquid containing molecules with nonpolar bonds?
(Multiple Choice)
4.8/5  (38)
(38)
What is the term for the number of water molecules in a hydrate?
(Multiple Choice)
4.9/5  (46)
(46)
Showing 141 - 160 of 180
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)