Exam 15: Electrolytes, Acids, and Bases
Exam 1: What Is Chemistry102 Questions
Exam 2: The Numerical Side of Chemistry201 Questions
Exam 3: The Evolution of Atomic Theory88 Questions
Exam 4: The Modern Model of the Atom144 Questions
Exam 5: Chemical Bonding and Nomenclature230 Questions
Exam 6: The Shape of Molecules138 Questions
Exam 7: Intermolecular Forces and the Phases of Matter107 Questions
Exam 8: Chemical Reactions122 Questions
Exam 9: Stoichiometry and the Mole241 Questions
Exam 10: Electron Transfer in Chemical Reactions138 Questions
Exam 11: What If There Were No Intermolecular Forces - the Ideal Gas118 Questions
Exam 12: Solutions143 Questions
Exam 13: When Reactants Turn Into Products106 Questions
Exam 14: Chemical Equilibrium135 Questions
Exam 15: Electrolytes, Acids, and Bases153 Questions
Exam 16: Nuclear Chemistry117 Questions
Exam 17: The Chemistry of Carbon105 Questions
Exam 18: Synthetic and Biological Polymers60 Questions
Select questions type
The conjugate acid of H₂PO₄- is ________.
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
A
Write down the chemical equation for the reaction of ammonia ( NH3 ) with water. In aqueous solution, what is the formula for conjugate acid of ammonia?
Free
(Short Answer)
4.7/5  (36)
(36)
Correct Answer:
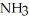 +
+ 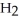 O →
O → 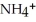 +
+ 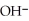 .
. 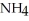 + is the conjugate acid of ammonia in aqueous solution.
+ is the conjugate acid of ammonia in aqueous solution.
The pH of natural rainwater is 5.6 due to the carbon dioxide in the atmosphere.
Free
(True/False)
4.8/5  (37)
(37)
Correct Answer:
True
Why is HF in water considered a weakly acidic solution, while HCl and HBr in water are considered strongly acidic solutions?
(Multiple Choice)
4.9/5  (40)
(40)
Identify each of the substances as an electrolyte or non-electrolyte.
-gasoline
(Multiple Choice)
4.7/5  (38)
(38)
Approximately one out of every 555 million water molecules autoionize.
(True/False)
5.0/5  (36)
(36)
Which of the following acids does not produce two H₃O+ ion per molecule of acid when dissociated in water?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following acids is a diprotic acid when dissolved in water?
(Multiple Choice)
4.8/5  (32)
(32)
Match each of the acids with the maximum number of H?O+ produced per molecule of acid.
-carbonic acid
(Multiple Choice)
4.7/5  (31)
(31)
An aqueous solution of pH 9.5 is more basic than an aqueous solution of pH 8.2.
(True/False)
4.8/5  (33)
(33)
Which of the following acids produces more than one H₃O+ ion per molecule of acid when dissociated in water?
(Multiple Choice)
4.8/5  (34)
(34)
Identify each of the substances as an electrolyte or non-electrolyte.
-solid sodium chloride
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 153
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)