Exam 15: Electrolytes, Acids, and Bases
Exam 1: What Is Chemistry102 Questions
Exam 2: The Numerical Side of Chemistry201 Questions
Exam 3: The Evolution of Atomic Theory88 Questions
Exam 4: The Modern Model of the Atom144 Questions
Exam 5: Chemical Bonding and Nomenclature230 Questions
Exam 6: The Shape of Molecules138 Questions
Exam 7: Intermolecular Forces and the Phases of Matter107 Questions
Exam 8: Chemical Reactions122 Questions
Exam 9: Stoichiometry and the Mole241 Questions
Exam 10: Electron Transfer in Chemical Reactions138 Questions
Exam 11: What If There Were No Intermolecular Forces - the Ideal Gas118 Questions
Exam 12: Solutions143 Questions
Exam 13: When Reactants Turn Into Products106 Questions
Exam 14: Chemical Equilibrium135 Questions
Exam 15: Electrolytes, Acids, and Bases153 Questions
Exam 16: Nuclear Chemistry117 Questions
Exam 17: The Chemistry of Carbon105 Questions
Exam 18: Synthetic and Biological Polymers60 Questions
Select questions type
Match each of the acids with the maximum number of H?O+ produced per molecule of acid.
-sulfurous acid
(Multiple Choice)
4.7/5  (38)
(38)
The carbonate ( 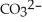 )/bicarbonate (
)/bicarbonate ( 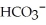 ) pair represents a base and its conjugate acid.
) pair represents a base and its conjugate acid.
(True/False)
4.8/5  (37)
(37)
The pH of an aqueous solution is 9.35. What is the pOH? (pH + pOH = 14 for aqueous acid/base systems).
(Multiple Choice)
4.8/5  (41)
(41)
Identify each of the substances as an electrolyte or non-electrolyte.
-tap water
(Multiple Choice)
4.8/5  (33)
(33)
Identify each of the acids as a strong acid or a weak acid.
-sulfuric acid
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following compounds qualifies as a Bronsted-Lowry base?
(Multiple Choice)
4.7/5  (49)
(49)
The pH of an aqueous solution of acetic acid is 3.76. What is the concentration of OH - in this solution?
(Essay)
4.8/5  (33)
(33)
Which of the following compounds is a weak electrolyte when dissolved in distilled water?
(Multiple Choice)
4.8/5  (32)
(32)
Identify each of the acids as a strong acid or a weak acid.
-nitric acid
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following compounds can behave both as a Bronsted-Lowry acid and a Bronsted-Lowry base?
(Multiple Choice)
4.8/5  (37)
(37)
What were the defining characteristics of bases as known to the alchemists?
(Short Answer)
4.9/5  (43)
(43)
Sodium chloride (NaCl) is considered an electrolyte because it dissolves in water into positive and negative ions.
(True/False)
4.8/5  (38)
(38)
The base in the forward reaction is ________. NH₃ + H₂O → NH4+ + OH-
(Multiple Choice)
4.9/5  (32)
(32)
Showing 21 - 40 of 153
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)