Exam 15: Infrared Spectroscopy and Mass Spectrometry
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties191 Questions
Exam 2: Molecular Representations154 Questions
Exam 3: Acids and Bases126 Questions
Exam 4: Alkanes and Cycloalkanes114 Questions
Exam 5: Stereoisomerism125 Questions
Exam 6: Chemical Reactivity and Mechanisms110 Questions
Exam 7: Substitution Reactions123 Questions
Exam 8: Alkenes: Structure and Preparation Via Elimination Reactions111 Questions
Exam 9: Addition Reactions of Alkenes148 Questions
Exam 10: Alkynes166 Questions
Exam 11: Radical Reactions90 Questions
Exam 12: Synthesis95 Questions
Exam 13: Alcohols and Phenols119 Questions
Exam 14: Ethers and Epoxides; Thiols and Sulfides130 Questions
Exam 15: Infrared Spectroscopy and Mass Spectrometry129 Questions
Exam 16: Nuclear Magnetic Resonance Spectroscopy114 Questions
Exam 17: Conjugated Pi Systems and Pericyclic Reactions131 Questions
Exam 18: Aromatic Compounds98 Questions
Exam 19: Aromatic Substitution Reactions109 Questions
Exam 20: Aldehydes and Ketones143 Questions
Exam 21: Carboxylic Acids and Their Derivatives117 Questions
Exam 22: Alpha Carbon Chemistry: Enols and Enolates131 Questions
Exam 23: Amines97 Questions
Exam 24: Carbohydrates122 Questions
Exam 25: Amino Acids, Peptides, and Proteins115 Questions
Exam 26: Lipids102 Questions
Exam 27: Synthetic Polymers100 Questions
Select questions type
Predict the product for the following reaction and explain how you will use IR spectroscopy to monitor the progress of the reaction. 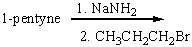
Free
(Essay)
4.9/5  (38)
(38)
Correct Answer:
CH3CH2CH2C CCH2CH2CH3
Absorptions for spC-H around 3300 cm-1 and C C around 2150 cm-1 should disappear from the product IR spectrum.
Propose possible structure(s) for a compound with molecular formula C4H7N that shows absorption at 2250 cm-1 in its IR spectrum.
Free
(Essay)
4.8/5  (38)
(38)
Correct Answer:
CH3CH2CH2C N OR (CH3)2CH2C N
Which of the following electromagnetic radiation has the longest wavelength?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
D
Which of the following compounds will have the lowest wavenumber for carbonyl absorption? 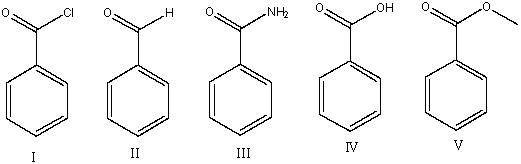
(Multiple Choice)
4.9/5  (33)
(33)
Propose a possible structure for a compound with molecular formula C6H5Br.
(Essay)
4.9/5  (32)
(32)
For which of the following compounds will the (M+2)+ . peak intensity be around one third the intensity of the molecular ion peak?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following mass spectra shows the presence of chlorine in a compound? 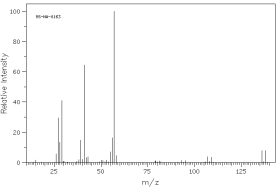
 I II
I II 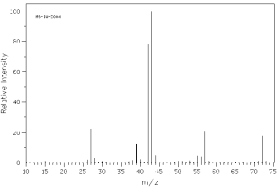
 III IV Four spectra above: courtesy of SDBS: National Institute of Advanced Industrial Science and Technology
III IV Four spectra above: courtesy of SDBS: National Institute of Advanced Industrial Science and Technology
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following alkene groups will produce the stronger signal? Explain why. 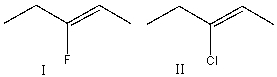
(Essay)
4.8/5  (40)
(40)
Which of the following is not true about the molecular ion in mass spectrometry?
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following will produce M+ . and (M+2)+ . peaks of equal intensity?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following is the base peak in the mass spectrum of 2,2,4-trimethylpentane?
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following compounds will show absorptions at 1640 cm-1, 2950 cm-1 and 3050 cm-1 on the IR spectrum? 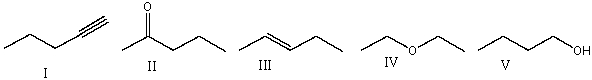
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following mass spectra shows the presence of bromine in a compound? 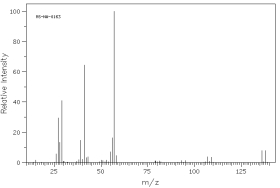
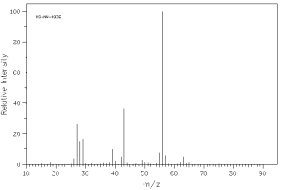 I II
I II 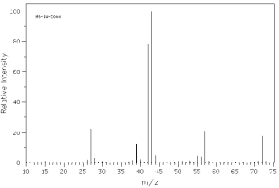
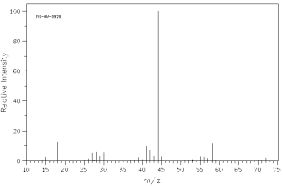 III IV Four spectra above: courtesy of SDBS: National Institute of Advanced Industrial Science and Technology
III IV Four spectra above: courtesy of SDBS: National Institute of Advanced Industrial Science and Technology
(Multiple Choice)
4.8/5  (44)
(44)
For the following pair of compounds the expected stretching absorption of the C=O bond is 1685cm-1 & 1715cm-1 respectively. Explain using both words and structural drawings. 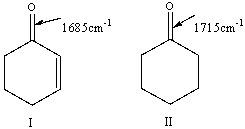
(Essay)
4.8/5  (37)
(37)
The separation of ions in the mass spectrometer is done by their ______.
(Multiple Choice)
4.9/5  (48)
(48)
For the following reaction, which of the following change(s) in the IR spectrum is consistent with conversion of the reactant to the product? 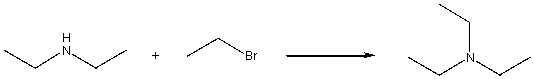
(Multiple Choice)
4.7/5  (32)
(32)
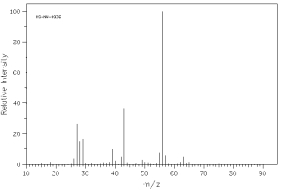
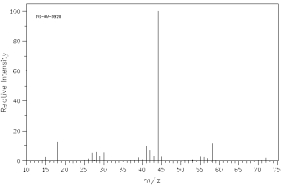
Which one of the following compounds is consistent with the mass spectrum below? 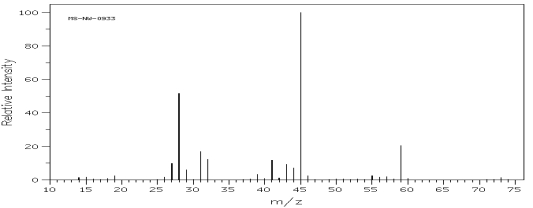 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology
(Multiple Choice)
4.9/5  (41)
(41)
Rank absorption of the indicated bonds in decreasing (highest to lowest) order of wavenumber. 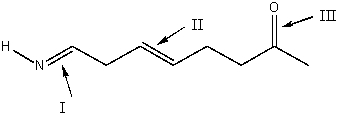
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following statement(s) is(are) true about the frequency of a stretching vibration according to Hooke's law?
(Multiple Choice)
4.8/5  (39)
(39)
Diluted alcohols show a _____absorption around 3600cm-1, due to _____.
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 129
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)