Exam 11: Radical Reactions
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties191 Questions
Exam 2: Molecular Representations154 Questions
Exam 3: Acids and Bases126 Questions
Exam 4: Alkanes and Cycloalkanes114 Questions
Exam 5: Stereoisomerism125 Questions
Exam 6: Chemical Reactivity and Mechanisms110 Questions
Exam 7: Substitution Reactions123 Questions
Exam 8: Alkenes: Structure and Preparation Via Elimination Reactions111 Questions
Exam 9: Addition Reactions of Alkenes148 Questions
Exam 10: Alkynes166 Questions
Exam 11: Radical Reactions90 Questions
Exam 12: Synthesis95 Questions
Exam 13: Alcohols and Phenols119 Questions
Exam 14: Ethers and Epoxides; Thiols and Sulfides130 Questions
Exam 15: Infrared Spectroscopy and Mass Spectrometry129 Questions
Exam 16: Nuclear Magnetic Resonance Spectroscopy114 Questions
Exam 17: Conjugated Pi Systems and Pericyclic Reactions131 Questions
Exam 18: Aromatic Compounds98 Questions
Exam 19: Aromatic Substitution Reactions109 Questions
Exam 20: Aldehydes and Ketones143 Questions
Exam 21: Carboxylic Acids and Their Derivatives117 Questions
Exam 22: Alpha Carbon Chemistry: Enols and Enolates131 Questions
Exam 23: Amines97 Questions
Exam 24: Carbohydrates122 Questions
Exam 25: Amino Acids, Peptides, and Proteins115 Questions
Exam 26: Lipids102 Questions
Exam 27: Synthetic Polymers100 Questions
Select questions type
Which of the following are major products of the reaction shown? 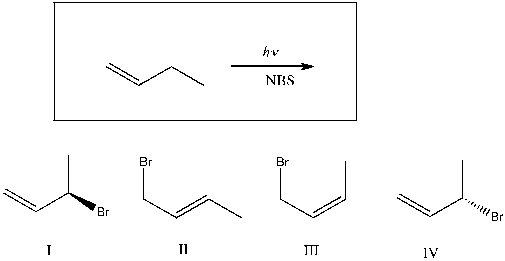
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
E
Which of the following shows the initiation step of monochlorination of methane? 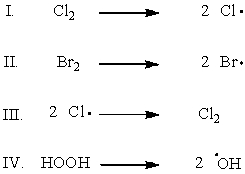
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
A
Predict the major product(s) of the following reaction. 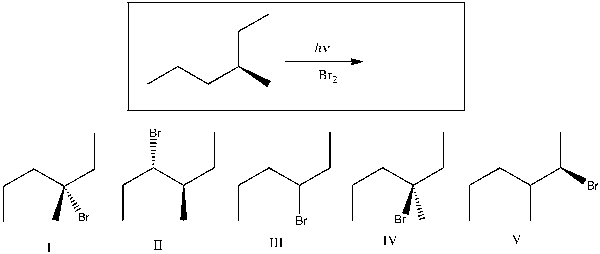
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
D
How many constitutional isomers are possible if propane is dichlorinated? Draw them.
(Essay)
4.8/5  (29)
(29)
Use correct arrow formalism to show the mechanism of the following radical process: 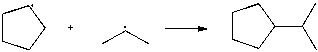
(Essay)
4.9/5  (35)
(35)
In the molecule shown below, determine which of the highlighted C-H bonds (from a to e) is expected to have the lowest bond dissociation energy. 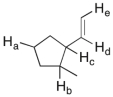
(Multiple Choice)
4.9/5  (38)
(38)
Which term most accurately describes the process shown below? 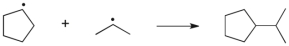
(Multiple Choice)
4.8/5  (37)
(37)
Propose an efficient synthesis of 3,4-dimethyl-2-pentanol using 2-methyl-2-butene and ethanol as your sources of carbon.
(Essay)
4.8/5  (41)
(41)
Which of the following compounds would be expected to be least destructive to the ozone layer?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following is expected to function as an antioxidant? 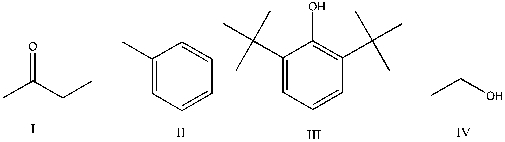
(Multiple Choice)
4.7/5  (37)
(37)
Draw the major product(s) of the following reaction. Is the product optically active? Explain. 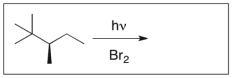
(Essay)
4.9/5  (35)
(35)
Use correct arrow formalism to draw all of the reasonable resonance structures for the radical shown below. 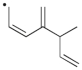
(Essay)
4.7/5  (34)
(34)
Propylene (propene) undergoes free radical polymerization with benzoyl peroxide, but does not produce very long chains. Provide a reasonable explanation for this result.
(Essay)
4.9/5  (40)
(40)
Which of the following represents a propagation step in the monochlorination of methylene chloride (CH2Cl2)?
(Multiple Choice)
4.7/5  (24)
(24)
Free radical chlorination of ethane can produce higher halogenation products (dichlorinated, trichlorinated, etc…) in addition to chloroethane. How could the production of higher halogenated products be minimized?
(Multiple Choice)
4.9/5  (33)
(33)
Upon treatment with NBS and irradiation with UV light, 1-ethyl-4-methylbenzene produces exactly three monobrominated compounds (including stereoisomers). Draw the products of this reaction.
(Essay)
4.9/5  (35)
(35)
Rank the following radicals in order of decreasing stability (most stable to least stable). 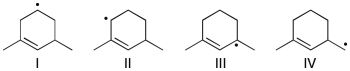
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following processes is responsible for the fact that free radical bromination of methane is slower than free radical chlorination?
(Multiple Choice)
4.9/5  (24)
(24)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)