Exam 15: Infrared Spectroscopy and Mass Spectrometry
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties191 Questions
Exam 2: Molecular Representations154 Questions
Exam 3: Acids and Bases126 Questions
Exam 4: Alkanes and Cycloalkanes114 Questions
Exam 5: Stereoisomerism125 Questions
Exam 6: Chemical Reactivity and Mechanisms110 Questions
Exam 7: Substitution Reactions123 Questions
Exam 8: Alkenes: Structure and Preparation Via Elimination Reactions111 Questions
Exam 9: Addition Reactions of Alkenes148 Questions
Exam 10: Alkynes166 Questions
Exam 11: Radical Reactions90 Questions
Exam 12: Synthesis95 Questions
Exam 13: Alcohols and Phenols119 Questions
Exam 14: Ethers and Epoxides; Thiols and Sulfides130 Questions
Exam 15: Infrared Spectroscopy and Mass Spectrometry129 Questions
Exam 16: Nuclear Magnetic Resonance Spectroscopy114 Questions
Exam 17: Conjugated Pi Systems and Pericyclic Reactions131 Questions
Exam 18: Aromatic Compounds98 Questions
Exam 19: Aromatic Substitution Reactions109 Questions
Exam 20: Aldehydes and Ketones143 Questions
Exam 21: Carboxylic Acids and Their Derivatives117 Questions
Exam 22: Alpha Carbon Chemistry: Enols and Enolates131 Questions
Exam 23: Amines97 Questions
Exam 24: Carbohydrates122 Questions
Exam 25: Amino Acids, Peptides, and Proteins115 Questions
Exam 26: Lipids102 Questions
Exam 27: Synthetic Polymers100 Questions
Select questions type
Which of the following wavenumber corresponds to the functional group region on an IR spectrum?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following information is primarily obtained from infrared spectroscopy?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following m/z values corresponds to the base peak for 2-chloro-2-methylpropane?
(Multiple Choice)
4.8/5  (33)
(33)
Arrange the following electromagnetic radiation in decreasing (highest to lowest) order of frequency. 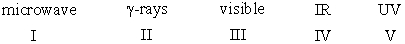
(Multiple Choice)
4.8/5  (30)
(30)
Which one of the following compounds is consistent with the following IR spectrum? 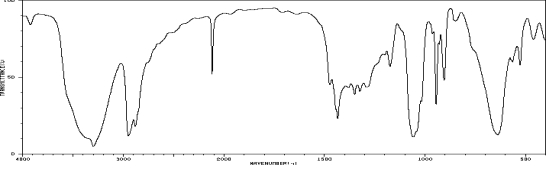 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 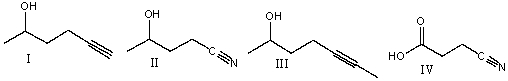
(Multiple Choice)
4.7/5  (44)
(44)
Rank absorption of the indicated bonds in decreasing (highest to lowest) order of wavenumber. 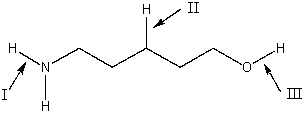
(Multiple Choice)
4.7/5  (34)
(34)
Which molecular formula is consistent with the following mass spectrum data? M+ . at m/z= 78, relative height=23.5%
(M+1)+ . at m/z= 79, relative height=0.78%
(M+2)+ . at m/z= 80, relative height=7.5%
(Multiple Choice)
5.0/5  (47)
(47)
Which one of the following compounds is consistent with the following IR spectrum? 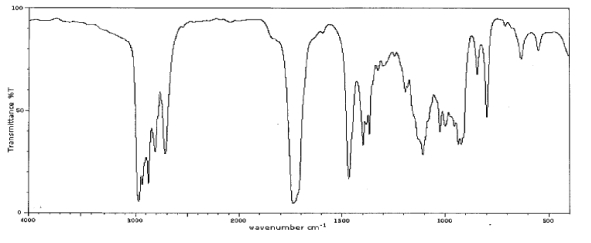 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 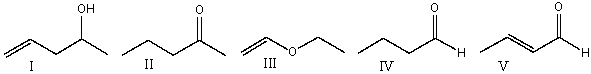
(Multiple Choice)
4.8/5  (44)
(44)
How will you distinguish between the following compounds using high-resolution mass spectrometry? 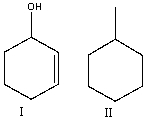
(Essay)
4.8/5  (29)
(29)
Which molecular formula is consistent with the following mass spectrum data? M+ . at m/z= 72, relative height=73.0%
(M+1)+ . at m/z= 73, relative height=3.3%
(Multiple Choice)
4.9/5  (33)
(33)
In mass spectrometry using electron impact ionization technique, a beam of high-energy electrons initially ejects one electron from the compound being studied. This produces a positively charged ion called the ____________________.
(Short Answer)
4.8/5  (34)
(34)
Primary amines show two medium absorption bands around 3400 cm-1, due to _______.
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following compounds will have a base peak at m/z=43?
(Multiple Choice)
4.8/5  (33)
(33)
Predict the product for the following reaction and explain how you will use IR spectroscopy to monitor the progress of the reaction. 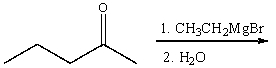
(Essay)
4.8/5  (34)
(34)
Which of the following alkene groups will produce the stronger signal? Explain why. 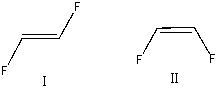
(Essay)
4.8/5  (38)
(38)
For which of the following compounds will the (M+2)+ . peak intensity be equal to the intensity of the molecular ion peak?
(Multiple Choice)
4.8/5  (36)
(36)
A compound with molecular formula C4H8O2, shows absorptions at 2200-3600 cm-1 (broad), 1720 cm-1 and at 1200 cm-1 on the IR spectrum. Propose a possible structure for this compound.
(Essay)
4.8/5  (41)
(41)
For the following pair of compounds the expected stretching absorption of the C=O bond is 1685cm-1 & 1655cm-1 respectively. Explain using both words and structural drawings. 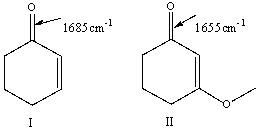
(Essay)
4.8/5  (41)
(41)
Which of the following is always true about the base peak in a mass spectrum?
(Multiple Choice)
4.7/5  (47)
(47)
Rank absorption of the indicated bonds in decreasing (highest to lowest) order of wavenumber. 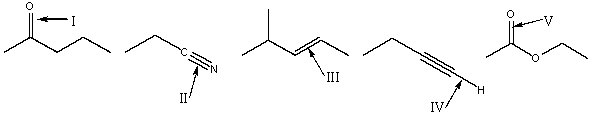
(Multiple Choice)
4.9/5  (35)
(35)
Showing 81 - 100 of 129
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)