Exam 11: Radical Reactions
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties191 Questions
Exam 2: Molecular Representations154 Questions
Exam 3: Acids and Bases126 Questions
Exam 4: Alkanes and Cycloalkanes114 Questions
Exam 5: Stereoisomerism125 Questions
Exam 6: Chemical Reactivity and Mechanisms110 Questions
Exam 7: Substitution Reactions123 Questions
Exam 8: Alkenes: Structure and Preparation Via Elimination Reactions111 Questions
Exam 9: Addition Reactions of Alkenes148 Questions
Exam 10: Alkynes166 Questions
Exam 11: Radical Reactions90 Questions
Exam 12: Synthesis95 Questions
Exam 13: Alcohols and Phenols119 Questions
Exam 14: Ethers and Epoxides; Thiols and Sulfides130 Questions
Exam 15: Infrared Spectroscopy and Mass Spectrometry129 Questions
Exam 16: Nuclear Magnetic Resonance Spectroscopy114 Questions
Exam 17: Conjugated Pi Systems and Pericyclic Reactions131 Questions
Exam 18: Aromatic Compounds98 Questions
Exam 19: Aromatic Substitution Reactions109 Questions
Exam 20: Aldehydes and Ketones143 Questions
Exam 21: Carboxylic Acids and Their Derivatives117 Questions
Exam 22: Alpha Carbon Chemistry: Enols and Enolates131 Questions
Exam 23: Amines97 Questions
Exam 24: Carbohydrates122 Questions
Exam 25: Amino Acids, Peptides, and Proteins115 Questions
Exam 26: Lipids102 Questions
Exam 27: Synthetic Polymers100 Questions
Select questions type
Which of the following are possible termination steps in the chlorination of methane?
(Multiple Choice)
4.8/5  (47)
(47)
Use correct arrow formalism to show the second propagation step for the reaction of a chlorine radical with ozone.
(Essay)
4.9/5  (33)
(33)
Methylenecyclopentane (below) can be exposed to allylic bromination conditions to give multiple regioisomeric compounds, including Compound X. Compound X can then be hydrogenated to give Compound Y, which has only 4 distinct resonances in the (proton-decoupled) 13C NMR spectrum. What are the structures of compounds X and Y? 
(Essay)
4.7/5  (38)
(38)
Use correct arrow formalism to show a homolytic bond cleavage of ethane to produce two methyl radicals.
(Essay)
4.7/5  (36)
(36)
Which of the following is the rate-determining step in the free-radical bromination of methane?
(Multiple Choice)
4.7/5  (45)
(45)
Which intermediate leads to the major product for the reaction of 2-methyl-2-butene with hydrogen bromide? 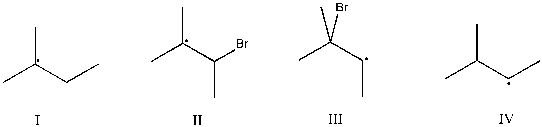
(Multiple Choice)
4.8/5  (37)
(37)
Compound A, C6H12 reacts with HBr/ROOR to give compound B, C6H13Br. Compound C, C6H14, reacts with bromine and light to produce compound B, C6H13Br. Suggest structures for compounds A, B, and C.
(Essay)
4.9/5  (36)
(36)
Compound A has molecular formula C9H20. Compound A produces exactly three constitutional isomers upon monochlorination, and one major constitutional isomer upon monobromination. Which of the following are possible structures of compound A? 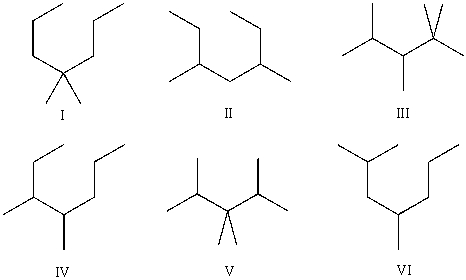
(Short Answer)
4.7/5  (42)
(42)
Your textbook mentions that the free radical polymerization of ethylene can produce branches. Although branches of various lengths are possible, butyl branches are very common. Suggest an explanation for this fact.
(Essay)
4.9/5  (33)
(33)
Propose an efficient sequence of reactions to accomplish the following transformation. 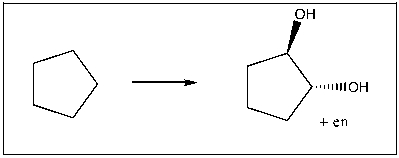
(Essay)
4.9/5  (34)
(34)
Upon treatment with NBS and irradiation with UV light, 2-propyl-1-pentene produces exactly four monobrominated compounds (including stereoisomers). Draw and name the products of this reaction.
(Essay)
4.9/5  (34)
(34)
Which monomer is used for the synthesis of poly(vinyl chloride)?
(Multiple Choice)
4.9/5  (40)
(40)
Which intermediate leads to the major product for the reaction of 2-methyl-2-butene with hydrogen bromide and hydrogen peroxide? 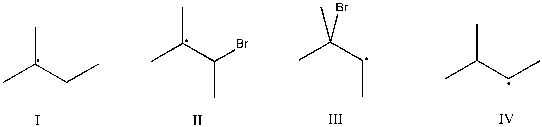
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following is (are) the likely major product(s) of the reaction shown? 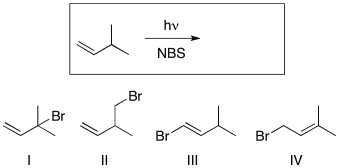
(Multiple Choice)
4.8/5  (41)
(41)
A bromine radical can add to the bond of 2-methylpropene. Use correct arrow formalism to show this process and the expected result.
(Essay)
4.8/5  (44)
(44)
Showing 21 - 40 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)