Exam 8: Alkenes: Structure and Preparation Via Elimination Reactions
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties191 Questions
Exam 2: Molecular Representations154 Questions
Exam 3: Acids and Bases126 Questions
Exam 4: Alkanes and Cycloalkanes114 Questions
Exam 5: Stereoisomerism125 Questions
Exam 6: Chemical Reactivity and Mechanisms110 Questions
Exam 7: Substitution Reactions123 Questions
Exam 8: Alkenes: Structure and Preparation Via Elimination Reactions111 Questions
Exam 9: Addition Reactions of Alkenes148 Questions
Exam 10: Alkynes166 Questions
Exam 11: Radical Reactions90 Questions
Exam 12: Synthesis95 Questions
Exam 13: Alcohols and Phenols119 Questions
Exam 14: Ethers and Epoxides; Thiols and Sulfides130 Questions
Exam 15: Infrared Spectroscopy and Mass Spectrometry129 Questions
Exam 16: Nuclear Magnetic Resonance Spectroscopy114 Questions
Exam 17: Conjugated Pi Systems and Pericyclic Reactions131 Questions
Exam 18: Aromatic Compounds98 Questions
Exam 19: Aromatic Substitution Reactions109 Questions
Exam 20: Aldehydes and Ketones143 Questions
Exam 21: Carboxylic Acids and Their Derivatives117 Questions
Exam 22: Alpha Carbon Chemistry: Enols and Enolates131 Questions
Exam 23: Amines97 Questions
Exam 24: Carbohydrates122 Questions
Exam 25: Amino Acids, Peptides, and Proteins115 Questions
Exam 26: Lipids102 Questions
Exam 27: Synthetic Polymers100 Questions
Select questions type
Which of the following is the correct mechanism for the elimination reaction of 1-bromo-2-methylcyclopentane with methoxide?
(Multiple Choice)
4.9/5  (44)
(44)
Draw the isomer of 2-bromo-1,1,3-trimethylcyclohexane that would be more reactive in an E2 elimination.
(Essay)
4.9/5  (32)
(32)
Which of the following alkyl halides would afford the indicated product upon reaction with sodium ethoxide?
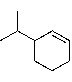
(Multiple Choice)
4.7/5  (36)
(36)
For the following dehydration, draw the structure of the intermediate carbocation. 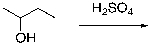
(Essay)
4.7/5  (31)
(31)
For the following dehydration, draw the structure of the intermediate carbocation. 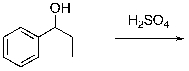
(Essay)
4.7/5  (32)
(32)
Viridenomycin, shown below, is a polyene antibiotic that displays anti-tumor activity. Its structural complexity and chemical instability have made it a challenging target for organic chemists to synthesize. Ignoring the benzene ring, how many disubstituted Z alkenes are present in viridenomycin? 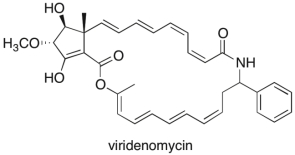
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following is the IUPAC name for the following compound? 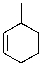
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following is the energy diagram for the following reaction? 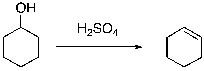
(Multiple Choice)
4.9/5  (38)
(38)
What would be the best base for performing the following elimination? 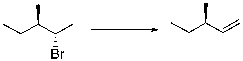
(Short Answer)
4.7/5  (32)
(32)
A student runs an elimination reaction in lab, beginning with 1-iodo-1-methyl cyclohexane. If the product obtained shows exactly 5 distinct carbons in the (proton-decoupled) 13C NMR spectrum, what base could have been used to cause the elimination?
(Short Answer)
4.8/5  (36)
(36)
Drawn below is the structure of Crestor® (rosuvastatin), a medication used to reduce cholesterol. What is the degree of substitution of the alkene of Crestor®? 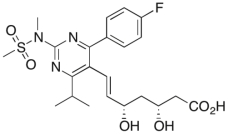
(Multiple Choice)
5.0/5  (39)
(39)
Showing 61 - 80 of 111
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)