Exam 3: Acids and Bases
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties191 Questions
Exam 2: Molecular Representations154 Questions
Exam 3: Acids and Bases126 Questions
Exam 4: Alkanes and Cycloalkanes114 Questions
Exam 5: Stereoisomerism125 Questions
Exam 6: Chemical Reactivity and Mechanisms110 Questions
Exam 7: Substitution Reactions123 Questions
Exam 8: Alkenes: Structure and Preparation Via Elimination Reactions111 Questions
Exam 9: Addition Reactions of Alkenes148 Questions
Exam 10: Alkynes166 Questions
Exam 11: Radical Reactions90 Questions
Exam 12: Synthesis95 Questions
Exam 13: Alcohols and Phenols119 Questions
Exam 14: Ethers and Epoxides; Thiols and Sulfides130 Questions
Exam 15: Infrared Spectroscopy and Mass Spectrometry129 Questions
Exam 16: Nuclear Magnetic Resonance Spectroscopy114 Questions
Exam 17: Conjugated Pi Systems and Pericyclic Reactions131 Questions
Exam 18: Aromatic Compounds98 Questions
Exam 19: Aromatic Substitution Reactions109 Questions
Exam 20: Aldehydes and Ketones143 Questions
Exam 21: Carboxylic Acids and Their Derivatives117 Questions
Exam 22: Alpha Carbon Chemistry: Enols and Enolates131 Questions
Exam 23: Amines97 Questions
Exam 24: Carbohydrates122 Questions
Exam 25: Amino Acids, Peptides, and Proteins115 Questions
Exam 26: Lipids102 Questions
Exam 27: Synthetic Polymers100 Questions
Select questions type
Predict the product(s) for the following reaction and draw the curved arrow mechanism. 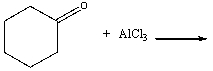
(Essay)
4.7/5  (36)
(36)
For the following reaction, identify the Lewis acid and the Lewis base and draw the curved arrow mechanism. 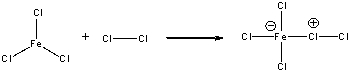
(Essay)
4.8/5  (41)
(41)
For the following acid-base reaction, predict which side the equilibrium is favored. 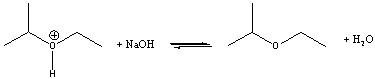
(Multiple Choice)
4.7/5  (38)
(38)
For the following reaction identify the acid and the base and predict the products. Draw the curved arrow mechanism for the formation of products and predict the direction of the equilibrium. 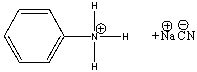
(Essay)
4.9/5  (41)
(41)
As a result of the "leveling effect," the strongest acid that can exist in appreciable concentration in aqueous solution is ____.
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following compounds is more acidic? Explain why.
CH3OH and CH3NH2
(Essay)
4.9/5  (36)
(36)
Tryptophan, an essential amino acid, is important in the synthesis of the neurotransmitter serotonin in the body. It has the following structure. Circle the three most acidic protons in tryptophan. 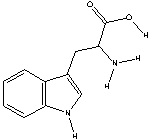
(Essay)
4.8/5  (37)
(37)
Which of the following solvents can be used with (CH3)3CLi?
(Multiple Choice)
4.8/5  (33)
(33)
For the following acid-base reaction, predict which side the equilibrium is favored. Explain why. 
(Essay)
4.7/5  (28)
(28)
A gain of proton by a Brønsted-Lowry base results in a ___.
(Multiple Choice)
4.9/5  (38)
(38)
Histidine an amino acid has the following structure. Circle the three most acidic protons in histidine. 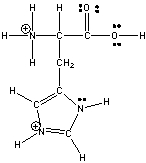
(Essay)
4.9/5  (38)
(38)
Which of the following solvents can not be used with (CH3)3COK?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 81 - 100 of 126
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)