Exam 12: Infrared Spectroscopy and Mass Spectrometry
Exam 1: Introduction and Review127 Questions
Exam 2: Structure and Properties of Organic Molecules129 Questions
Exam 3: Structure and Stereochemistry of Alkanes129 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry128 Questions
Exam 6: Alkyl Halides: Nucleophilic Substitution and Elimination132 Questions
Exam 7: Structure and Synthesis of Alkenes128 Questions
Exam 8: Reactions of Alkenes132 Questions
Exam 9: Alkynes124 Questions
Exam 10: Structure and Synthesis of Alcohols132 Questions
Exam 11: Reactions of Alcohols124 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry120 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes132 Questions
Exam 19: Amines129 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives128 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds125 Questions
Exam 23: Carbohydrates and Nucleic Acids125 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
How could IR spectroscopy be used to distinguish between the following pair of compounds?
CH2=CHCH2CH(CH3)2 and CH3CH2CH2CH(CH3)2
Free
(Short Answer)
4.7/5  (34)
(34)
Correct Answer:
C-C stretch around 1640 cm-1; vinylic CH stretch above 3000 cm-1
What wavelength in mm is equivalent to a wavenumber of 1750 cm-1?
Free
(Short Answer)
4.9/5  (39)
(39)
Correct Answer:
5.71 mm
Which compound would be expected to show intense IR absorption at 3300 cm-1?
Free
(Multiple Choice)
4.9/5  (29)
(29)
Correct Answer:
D
Rank the following bonds in order of increasing stretching frequency (cm-1) in IR spectroscopy: 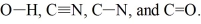
(Short Answer)
4.8/5  (34)
(34)
2-Methylhexane shows an intense peak in the mass spectrum at m/z = 43. Propose a likely structure for this fragment.
(Essay)
4.8/5  (35)
(35)
Absorption of what type of electromagnetic radiation results in transitions among allowed nuclear magnetic spin states?
(Multiple Choice)
4.8/5  (45)
(45)
3-Pentanol can be oxidized to 3-pentanone using sodium dichromate. How can the completeness of conversion be gauged using IR spectroscopy?
(Essay)
4.8/5  (41)
(41)
Which of the following structures is consistent with the IR spectra shown below? 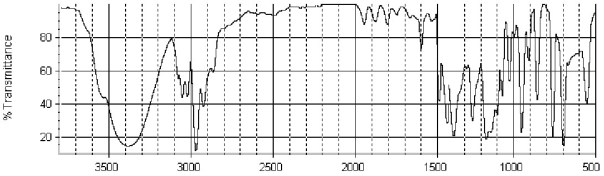
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following does not have a broad absorption with one or more spikes that is centered about 3300 cm-1 in the IR?
(Multiple Choice)
4.7/5  (37)
(37)
What IR absorption is characteristic of the O-H stretch in alcohols?
(Multiple Choice)
4.7/5  (33)
(33)
Deduce a possible structure for the compound with the IR absorptions below.
C3H5N: 2950, 2250 cm-1
(Short Answer)
4.8/5  (35)
(35)
The mass spectrum of alcohols often fail to exhibit detectable M peaks but instead show relatively large ________ peaks.
(Multiple Choice)
4.8/5  (38)
(38)
Provide an equation which relates the energy of a photon to its wavelength.
(Short Answer)
4.8/5  (46)
(46)
Absorption of what type of electromagnetic radiation results in transitions among allowed rotational motions?
(Multiple Choice)
4.8/5  (34)
(34)
Describe the fate of a molecule from introduction to detection in a mass spectrometer.
(Essay)
4.7/5  (34)
(34)
The mass spectrum of which compound has M+ and M+2 peaks of approximately equal intensity?
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following factors influences how intensely a functional group absorbs infrared radiation (large peak vs. small peak)?
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following structures is consistent with the IR spectra shown below? 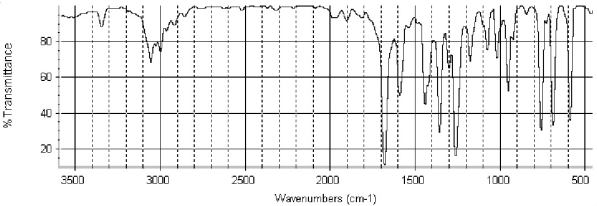
(Multiple Choice)
4.9/5  (40)
(40)
In an IR spectrometer, the ________ uses prisms or diffraction gratings to allow only one frequency of light to enter the detector at a time.
(Short Answer)
5.0/5  (28)
(28)
Which bond in the structure below would give rise to the highest absorption frequency in an IR spectrum? 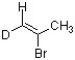
(Multiple Choice)
4.8/5  (39)
(39)
Showing 1 - 20 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)