Exam 1: Introduction and Review
Exam 1: Introduction and Review127 Questions
Exam 2: Structure and Properties of Organic Molecules129 Questions
Exam 3: Structure and Stereochemistry of Alkanes129 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry128 Questions
Exam 6: Alkyl Halides: Nucleophilic Substitution and Elimination132 Questions
Exam 7: Structure and Synthesis of Alkenes128 Questions
Exam 8: Reactions of Alkenes132 Questions
Exam 9: Alkynes124 Questions
Exam 10: Structure and Synthesis of Alcohols132 Questions
Exam 11: Reactions of Alcohols124 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry120 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes132 Questions
Exam 19: Amines129 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives128 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds125 Questions
Exam 23: Carbohydrates and Nucleic Acids125 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Draw a correct Lewis structure for boric acid, B(OH)3.
Free
(Essay)
4.8/5  (39)
(39)
Correct Answer:
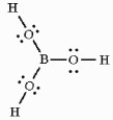 or
or 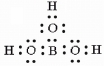
For most compounds in which a nitrogen atom bears no formal charge, the valence of this nitrogen atom is ________.
Free
(Short Answer)
4.9/5  (39)
(39)
Correct Answer:
3
Write a completed equation for the acid-base pair shown below.
HCN + NaOH →
Free
(Short Answer)
4.9/5  (33)
(33)
Correct Answer:
HCN + NaOH → NaCN + H2O
Provide the line-angle formula (skeletal structure) for CH2=CHCH2CH2C(CH3)3.
(Essay)
4.8/5  (43)
(43)
Draw the line energy orbital diagram for the outer shell of an uncharge nitrogen atom and describe the location and number of unshared electrons.
(Essay)
4.9/5  (30)
(30)
Write a completed equation for the acid-base pair shown below.
HCO2H + -NH2 →
(Essay)
4.9/5  (34)
(34)
Nitroamines are common functional groups found in energetic materials, such as RDX and HMX. For the structure below, draw two other significant resonance structures, include any formal charges, and indicate the hybridization on each nitrogen and oxygen. 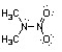
(Essay)
4.9/5  (40)
(40)
Provide the products of the following acid-base reaction.
(CH3)3NH+ + HO-→
(Essay)
4.7/5  (36)
(36)
Which sequence correctly ranks the following protons in order of increasing pKa value? 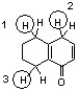
(Multiple Choice)
4.8/5  (36)
(36)
The compound phenol is shown below. Provide the structure of the conjugate base of phenol. 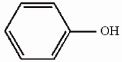
(Essay)
4.9/5  (36)
(36)
Provide the line-angle formula (skeletal structure) for (CH3CH2)2C=O.
(Essay)
4.9/5  (39)
(39)
Which of the following molecules contains a polar covalent bond?
(Multiple Choice)
4.8/5  (26)
(26)
The pH of a 150 mL aqueous solution of 2.13 x 10-3 M HCl is ________.
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)