Exam 4: The Study of Chemical Reactions
Exam 1: Introduction and Review127 Questions
Exam 2: Structure and Properties of Organic Molecules129 Questions
Exam 3: Structure and Stereochemistry of Alkanes129 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry128 Questions
Exam 6: Alkyl Halides: Nucleophilic Substitution and Elimination132 Questions
Exam 7: Structure and Synthesis of Alkenes128 Questions
Exam 8: Reactions of Alkenes132 Questions
Exam 9: Alkynes124 Questions
Exam 10: Structure and Synthesis of Alcohols132 Questions
Exam 11: Reactions of Alcohols124 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry120 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes132 Questions
Exam 19: Amines129 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives128 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds125 Questions
Exam 23: Carbohydrates and Nucleic Acids125 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Does one expect ΔS° in a propagation step of the free-radical chlorination of methane to be greater than zero, less than zero, or approximately equal to zero? Briefly explain your choice.
Free
(Essay)
4.9/5  (40)
(40)
Correct Answer:
The propagation steps of the free-radical chlorination of methane are shown
below.
CH4 + Cl∙ → CH3∙ + H-Cl
CH3∙ + Cl-Cl → CH3Cl + Cl∙
In each of the steps, two reactant molecules generate two product molecules. This similarity in the number of molecular species means that the disorder in the reaction is neither greatly increased nor diminished. Therefore, one expects ΔS° in either propagation step to be approximately equal to zero.
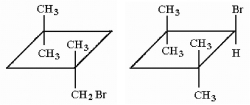
Write the structures of all of the monobromination products of 1,1,3,3-tetramethylcyclobutane.
Free
(Essay)
4.8/5  (46)
(46)
Correct Answer:
Consider the three-step mechanism for the reaction of A through intermediates B and C to produce D shown below.
A → B Ea = 15 kcal/mol, ΔH° = 13 kcal/mol
B → C Ea = 10 kcal/mol, ΔH° = -6 kcal/mol
C → D Ea = 2 kcal/mol, ΔH° = -20 kcal/mol
What's the enthalpy difference between reactant A and intermediate C?
Free
(Short Answer)
4.9/5  (40)
(40)
Correct Answer:
+7 kcal/mol
Which of the following correctly expresses the standard Gibbs free energy change of a reaction in terms of the reaction temperature (T) and equilibrium constant (K)?
(Multiple Choice)
4.8/5  (34)
(34)
Consider the following substitution reaction with a ΔG° value of -91.1 kJ/mole.
HO- + CH3Cl ↔ CH3OH + Cl-
Given this information which of the following statements must be true?
(R = 8.315 J/mole K)
(Multiple Choice)
4.8/5  (39)
(39)
Explain how the termination step in a free-radical chain reaction stops the chain.
(Essay)
4.9/5  (30)
(30)
What is the hybridization of the negatively charged carbon in (CH3)3C:-?
(Short Answer)
4.8/5  (44)
(44)
In the reaction of Cl2 with ethane and UV light, which of the following reactions would be a propagation event(s)?
I. Cl∙ + CH3-CH3 → CH3-CH2-Cl + H∙
II. Cl∙ + CH3-CH3 → CH3-H2C∙ + HCl
III. Cl∙ + CH3-H2C∙ → CH3-CH2-Cl
IV. Cl2 + CH3-H2C∙ → CH3-CH2-Cl + Cl∙
V. Cl2 + UV light → C l∙ + Cl∙
(Multiple Choice)
5.0/5  (40)
(40)
Given a K of 0.45 at 25°C, calculate the corresponding DG° in kJ/mol. [R = 8.314 J/K∙ mol]
(Short Answer)
4.8/5  (39)
(39)
How many secondary hydrogens are present in the hydrocarbon below? 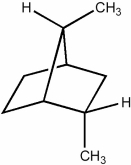
(Multiple Choice)
4.9/5  (40)
(40)
If the equilibrium constant (Keq) of a reaction is 0.5 then which of the following that must be true?
(Multiple Choice)
4.9/5  (30)
(30)
What is the hybridization of the positively charged carbon in (CH3)3C+?
(Short Answer)
4.8/5  (28)
(28)
Predict the major monobromination product in the following reaction.
(CH3)3CCH2CH3 + Br2 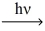
(Essay)
4.8/5  (38)
(38)
Which H atom in the molecule shown will be most readily abstracted by a bromine radical? 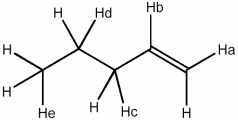
(Multiple Choice)
4.7/5  (47)
(47)
When the reaction between methane and chlorine is photochemically initiated, which of the following compounds be formed through a termination reaction?
(Multiple Choice)
4.7/5  (39)
(39)
The rate of a reaction typically increases as the temperature increases because:
(Multiple Choice)
4.8/5  (28)
(28)
The relative reactivity of the 1°: 2°: 3° hydrogens of (CH3)3CCH2CH3 in free radical chlorination is 1: 3.8: 5.0. Provide the structure of each monochlorination product, and estimate the relative amount of each in the mixture of monochlorinated products.
(Essay)
4.9/5  (38)
(38)
Consider the transformation of A to B (i.e., A →
B). If at equilibrium at 25°C the concentration of A is 20% of the initial concentration of A, determine the value of ΔG° (in kcal/mol) for this reaction.
R = 1.987 cal/mol∙K.
(Short Answer)
4.9/5  (44)
(44)
Showing 1 - 20 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)