Exam 13: Physical Properties of Solutions
Exam 1: Chemistry: the Central Science129 Questions
Exam 2: Atoms Molecules and Ions120 Questions
Exam 3: Stoichiometry Ratios of Combination131 Questions
Exam 4: Reactions in Aqueous Solutions144 Questions
Exam 5: Thermochemistry134 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms107 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I Basic Concepts95 Questions
Exam 9: Chemical Bonding Ii Molecular Geometry and Bonding Theories137 Questions
Exam 10: Gases131 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids135 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions147 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium135 Questions
Exam 16: Acids and Bases133 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria129 Questions
Exam 18: Entropy Free Energy and Equilibrium61 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry125 Questions
Exam 21: Environmental Chemistry131 Questions
Exam 22: Coordination Chemistry128 Questions
Exam 23: Metallurgy and the Chemistry of Metals115 Questions
Exam 24: Nonmetallic Elements and Their Compounds119 Questions
Exam 25: Organic Chemistry148 Questions
Select questions type
Calculate the molality of a 20.0% by mass ammonium sulfate (NH4)2SO4 solution.The density of the solution is 1.117 g/mL.
(Multiple Choice)
4.8/5  (42)
(42)
Barbiturates are synthetic drugs used as sedatives and hypnotics.Barbital (184.2g/mol)is one of the simplest of these drugs.What is the boiling point of a solution prepared by dissolving 42.5 g of barbital in 825 g of acetic acid? (For pure acetic acid,Kb = 3.07°C/m; boiling point of pure acetic acid = 117.9°C)
(Multiple Choice)
4.9/5  (43)
(43)
Gases with high boiling points tend to be more soluble in water than ones with low boiling points.
(True/False)
4.8/5  (35)
(35)
What is the value for the van't Hoff factor for all nonelectrolytes?
(Multiple Choice)
4.8/5  (45)
(45)
What is the name given to a solution that contains less solute than it has the capacity to dissolve?
(Multiple Choice)
4.8/5  (36)
(36)
Which one of the following substances is the strongest electrolyte?
(Multiple Choice)
4.7/5  (37)
(37)
What is the freezing point of a solution made from 22.0 g of octane (C8H18)dissolved in 148.0 g of benzene? (For benzene,freezing point = 5.50°C; Kf = 5.12°C/m)
(Multiple Choice)
4.8/5  (28)
(28)
What is the molarity of a solution prepared by diluting 1.85 L of 6.5 M KOH to 11.0 L?
(Multiple Choice)
4.8/5  (33)
(33)
Calcium nitrite is used as a corrosion inhibitor in lubricants.What is the molality of a solution prepared by dissolving 18.5 g of calcium nitrite in 83.5 g of distilled water?
(Multiple Choice)
4.9/5  (34)
(34)
What is the percent CsCl by mass in a 0.711 M aqueous CsCl solution that has a density of 1.091g/mL?
(Multiple Choice)
4.8/5  (36)
(36)
The distinguishing characteristic of all nonelectrolyte solutions is that they
(Multiple Choice)
4.9/5  (42)
(42)
The osmotic pressure of a 0.010 M MgSO4 solution at 25°C is 0.318 atm.Calculate i,the van't Hoff factor,for this MgSO4 solution. (R = 0.08206 L • atm/K • mol)
(Multiple Choice)
4.9/5  (37)
(37)
Which substance is present in the largest proportion in a solution?
(Multiple Choice)
4.8/5  (35)
(35)
Below is a diagram representing a solvent A(l)in a 1-L beaker,and a solute X dissolved in the solvent.Solvent A has a density of 0.8 g/mL,and a molar mass of 40 g/mol. Solute X has a molar mass of 30 g/mol.Each circle of X represents 1 mol of X.Assume that the solute addition does not significantly change the volume of liquid in the beaker. 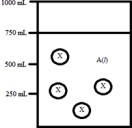 What is the mole fraction of the solute X in this solution?
What is the mole fraction of the solute X in this solution?
(Multiple Choice)
4.7/5  (37)
(37)
The mixing of solvent molecules and solute molecules is usually described as
(Multiple Choice)
4.8/5  (30)
(30)
What is defined as a solution that has a higher concentration of dissolved substances than plasma?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 21 - 40 of 147
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)