Exam 13: Physical Properties of Solutions
Exam 1: Chemistry: the Central Science129 Questions
Exam 2: Atoms Molecules and Ions120 Questions
Exam 3: Stoichiometry Ratios of Combination131 Questions
Exam 4: Reactions in Aqueous Solutions144 Questions
Exam 5: Thermochemistry134 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms107 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I Basic Concepts95 Questions
Exam 9: Chemical Bonding Ii Molecular Geometry and Bonding Theories137 Questions
Exam 10: Gases131 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids135 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions147 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium135 Questions
Exam 16: Acids and Bases133 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria129 Questions
Exam 18: Entropy Free Energy and Equilibrium61 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry125 Questions
Exam 21: Environmental Chemistry131 Questions
Exam 22: Coordination Chemistry128 Questions
Exam 23: Metallurgy and the Chemistry of Metals115 Questions
Exam 24: Nonmetallic Elements and Their Compounds119 Questions
Exam 25: Organic Chemistry148 Questions
Select questions type
Below is a diagram representing two chambers containing solvent A,separated by a semipermeable membrane (a dashed line)which allows only solvent A to pass through. 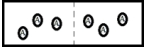 If a solute X is added to the chamber on the left,which diagram below best represents how the system will respond in order to reach a new equilibrium?
If a solute X is added to the chamber on the left,which diagram below best represents how the system will respond in order to reach a new equilibrium?
(Multiple Choice)
4.7/5  (32)
(32)
What mass of ethanol (C2H5OH),a nonelectrolyte,must be added to 10.0 L of water to give a solution that freezes at -10.0°C? Assume the density of water is 1.0 g/mL.(Kf of water is 1.86°C/m.)
(Multiple Choice)
5.0/5  (40)
(40)
If a 100-mL sample of a volatile liquid such as diethyl ether is introduced into a 250-mL
flask which is immediately sealed,the pressure inside will increase above atmospheric pressure.Explain.
(Essay)
4.9/5  (32)
(32)
What is the freezing point of a solution prepared from 50.0 g ethylene glycol (C2H6O2) and 85.0 g H2O?
(For water,Kf = 1.86°C/m)
(Multiple Choice)
4.9/5  (48)
(48)
Osmosis is the selective passage of solvent molecules through a porous membrane from a more concentrated solution to a more dilute one.
(True/False)
4.7/5  (39)
(39)
The density of a 20.3 M CH3OH (methanol)solution is 0.858 g/mL.What is the molality of this solution? H2O is the solvent.
(Multiple Choice)
4.9/5  (37)
(37)
Copper(II)bromide is used as a wood preservative.What mass of CuBr2 is needed to prepare 750.0 mL of a 1.25 M solution?
(Multiple Choice)
4.9/5  (31)
(31)
What is the name of the process in which an ion or a molecule is surrounded by solvent molecules arranged in a specific manner?
(Short Answer)
5.0/5  (39)
(39)
The ______________ ______________ is the scattered light that is caused by the dispersed phase of a colloid that has a beam of light passed through it.
(Short Answer)
4.8/5  (36)
(36)
______________ is the process used to stabilize a colloid that would otherwise not stay dispersed.
(Short Answer)
4.8/5  (32)
(32)
Below is a diagram representing a solvent A(l)in a 1-L beaker,and a solute X dissolved in the solvent.Solvent A has a density of 0.8 g/mL,and a molar mass of 40 g/mol. Solute X has a molar mass of 30 g/mol.Each circle of X represents 1 mol of X.Assume that the solute addition does not significantly change the volume of liquid in the beaker. 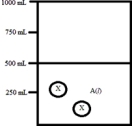 What is the molality of the solute X in this solution?
What is the molality of the solute X in this solution?
(Multiple Choice)
4.7/5  (29)
(29)
What is the percent CdSO4 by mass in a 1.00 molal aqueous CdSO4 solution?
(Multiple Choice)
4.8/5  (29)
(29)
Benzaldehyde (106.12 g/mol),also known as oil of almonds,is used in the manufacture of dyes and perfumes and in flavorings.What would be the freezing point of a solution prepared by dissolving 75.00 g of benzaldehyde in 850.0 g of ethanol? (For ethanol,Kf = 1.99°C/m; freezing point of pure ethanol = -117.3°C)
(Multiple Choice)
4.8/5  (35)
(35)
What mass of water is required to dissolve 25.31 g of potassium nitrate (KNO3)in order to prepare a 0.1982 m solution?
(Multiple Choice)
4.8/5  (41)
(41)
Potassium fluoride is used for frosting glass.Calculate the molarity of a solution prepared by dissolving 78.6 g of KF in enough water to produce 225 mL of solution.
(Multiple Choice)
4.8/5  (41)
(41)
Cadmium bromide is used in photography and lithography.What is the molality of a solution prepared by dissolving 45.38 g of CdBr2 in 375.0 g of water?
(Multiple Choice)
4.9/5  (28)
(28)
The osmotic pressure of a 0.82 M HCl solution is 35.9 atm at 18°C.Calculate the van't Hoff factor for HCl at this concentration.
(Multiple Choice)
4.7/5  (34)
(34)
Showing 121 - 140 of 147
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)