Multiple Choice
How much energy is evolved during the formation of 98.7 g of Fe according to the reaction below?
Fe2O3(s) + 2Al(s) → Al2O3(s) + 2Fe(s) 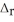 H° = -852 kJ
H° = -852 kJ
A) 753 kJ
B) 1.51 × 103 kJ
C) 4.20 × 103 kJ
D) 482 kJ
E) 241 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Use the information provided to determine <img
Q2: Given w = 0, an endothermic reaction
Q4: An unknown metal alloy, mass = 26.3
Q5: Identify an energy source that is non-renewable.<br>A)
Q6: How much heat is absorbed when 50.00
Q7: Calculate the final temperature of a 38.1
Q8: A sample of copper absorbs 43.6 kJ
Q9: Which of the following processes is endothermic?<br>A)
Q10: Use the standard reaction enthalpies given below
Q11: According to the following thermochemical equation, what