Multiple Choice
The reaction system 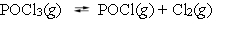 is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of chlorine is reduced by 50%?
is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of chlorine is reduced by 50%?
A) POCl3 will be consumed as equilibrium is established.
B) POCl will be consumed as equilibrium is established.
C) Chlorine will be consumed as equilibrium is established.
D) The partial pressure of POCl will decrease while the partial pressure of Cl2 increases as equilibrium is established.
E) The volume will have to decrease before equilibrium can be reestablished.
Correct Answer:

Verified
Correct Answer:
Verified
Q31: A good catalyst for a reaction will
Q59: At high temperatures, carbon reacts with O<sub>2</sub>
Q60: What is the mass-action expression, Q<sub>c</sub>, for
Q61: Write the mass-action expression, Q<sub>c</sub>, for the
Q62: What is the mass-action expression, Q<sub>p</sub>, for
Q65: What is the mass-action expression, Q<sub>c</sub>, for
Q66: A chemical reaction has an equilibrium constant
Q67: Ammonia is synthesized in the Haber process:
Q68: The reaction quotient, Q<sub>c</sub>, for a reaction
Q69: The equilibrium constant K<sub>c</sub> for the