Multiple Choice
What is the mass-action expression, Qp, for the following reaction? 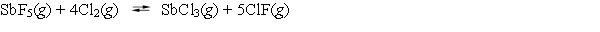
A) 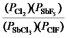
B) 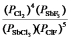
C) 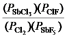
D) 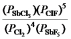
E) None of these choices is the correct mass-action expression.
Correct Answer:

Verified
Correct Answer:
Verified
Q31: A good catalyst for a reaction will
Q57: Carbon monoxide and chlorine combine in an
Q58: Nitrogen dioxide decomposes according to the
Q59: At high temperatures, carbon reacts with O<sub>2</sub>
Q60: What is the mass-action expression, Q<sub>c</sub>, for
Q61: Write the mass-action expression, Q<sub>c</sub>, for the
Q64: The reaction system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="The reaction
Q65: What is the mass-action expression, Q<sub>c</sub>, for
Q66: A chemical reaction has an equilibrium constant
Q67: Ammonia is synthesized in the Haber process: