Multiple Choice
Write the mass-action expression, Qc, for the following chemical reaction. 
A) 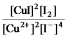
B) 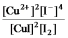
C) 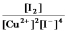
D) 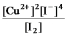
E) 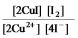
Correct Answer:

Verified
Correct Answer:
Verified
Q31: A good catalyst for a reaction will
Q56: Consider the reversible reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q57: Carbon monoxide and chlorine combine in an
Q58: Nitrogen dioxide decomposes according to the
Q59: At high temperatures, carbon reacts with O<sub>2</sub>
Q60: What is the mass-action expression, Q<sub>c</sub>, for
Q62: What is the mass-action expression, Q<sub>p</sub>, for
Q64: The reaction system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="The reaction
Q65: What is the mass-action expression, Q<sub>c</sub>, for
Q66: A chemical reaction has an equilibrium constant