Multiple Choice
At high temperatures, carbon reacts with O2 to produce CO as follows: 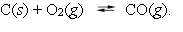 When 0.350 mol of O2 and excess carbon were placed in a 5.00-L container and heated, the equilibrium concentration of CO was found to be 0.060 M. What is the equilibrium constant, Kc, for this reaction?
When 0.350 mol of O2 and excess carbon were placed in a 5.00-L container and heated, the equilibrium concentration of CO was found to be 0.060 M. What is the equilibrium constant, Kc, for this reaction?
A) 0.010
B) 0.072
C) 0.090
D) 0.17
E) 1.2
Correct Answer:

Verified
Correct Answer:
Verified
Q31: A good catalyst for a reaction will
Q54: A mixture of 0.500 mole of carbon
Q55: The equilibrium constant, K<sub>p</sub>, for the
Q56: Consider the reversible reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q57: Carbon monoxide and chlorine combine in an
Q58: Nitrogen dioxide decomposes according to the
Q60: What is the mass-action expression, Q<sub>c</sub>, for
Q61: Write the mass-action expression, Q<sub>c</sub>, for the
Q62: What is the mass-action expression, Q<sub>p</sub>, for
Q64: The reaction system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="The reaction