Essay
The free energy of formation (ΔGf°) is defined analogously to a heat of formation: it is the standard free energy for formation of a compound from its elements in their standard states at 298 K. Consider the following reaction and their associated standard free energies of formation (all in kJ mol−1). Although these values are for the gas phase, they give us some idea of what to expect in solution.
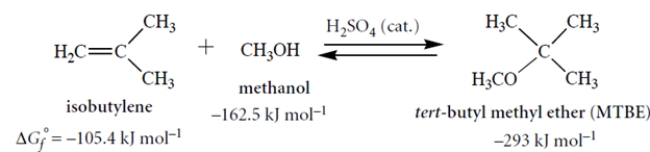 MTBE has been an important gasoline additive until it began to be replaced by ethanol in 2002.
MTBE has been an important gasoline additive until it began to be replaced by ethanol in 2002.
Calculate the equilibrium constant for this reaction in the left-to-right direction. Show your work.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Complete the electron-pair displacement reaction by showing
Q3: Aspirin has the structure on the left
Q4: Give the products of the electron-pair displacement
Q5: Using curved arrows, show how each resonance
Q6: Consider the acid-base equilibrium:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Consider
Q7: Show the curved-arrow notation and the product
Q8: Select the strongest acid.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Select the
Q9: Select the equilibrium that most favors
Q10: Choose the number that is closest to
Q11: Ascorbic acid (Vitamin C) is a diprotic