Essay
Aspirin has the structure on the left with a pKa of 3.4.
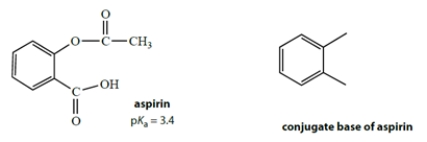 a. Complete the structure on the right for the conjugate base of aspirin.
a. Complete the structure on the right for the conjugate base of aspirin.
b. In a large excess of stomach acid at pH = 2, calculate the fraction of aspirin that is ionized (to 2 significant figures).
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Complete the electron-pair displacement reaction by showing
Q2: The free energy of formation (ΔG<sub>f</sub>°) is
Q4: Give the products of the electron-pair displacement
Q5: Using curved arrows, show how each resonance
Q6: Consider the acid-base equilibrium:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Consider
Q7: Show the curved-arrow notation and the product
Q8: Select the strongest acid.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Select the
Q9: Select the equilibrium that most favors
Q10: Choose the number that is closest to
Q11: Ascorbic acid (Vitamin C) is a diprotic