Multiple Choice
Which of the compounds would have the strongest C=C stretching absorption in its IR spectrum?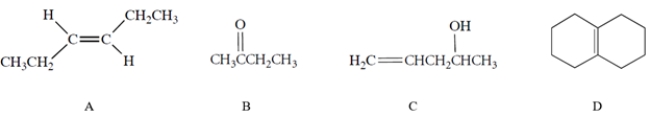
A) compound A
B) compound B
C) compound C
D) compound D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: The electron ionization mass spectrum of 1-hexanol
Q16: The IR absorption that occurs at the
Q17: These two compounds have similar molecular weights.
Q18: The electron ionization of an unknown neutral
Q19: A graduate student is cleaning out the
Q20: Typical IR absorptions occur in the 650-3700
Q21: When the epoxy thiol is treated with
Q22: The base peak of 2,2-dimethylpentane is at
Q23: Menthol is obtained from oils of peppermint
Q24: Select the true statement.<br>A) The S-H stretching