Essay
Consider this compound:
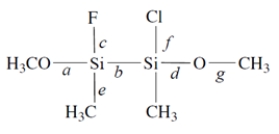 1. Of the labeled bonds, the longest bond is ________________.
1. Of the labeled bonds, the longest bond is ________________.
2. The most polar bond is ________________.
3. The bonding geometry at the silicon atoms is ________________.
4. The hybridization of the silicon atoms is ________________.
Correct Answer:

Verified
1. b
2. c
...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
2. c
...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q16: What is the geometry of the borohydride
Q17: Complete the structure for the cyanate ion
Q18: Given that the acetate anion has the
Q19: Add valence electrons to the structures so
Q20: Which compound has the largest dipole moment?<br><img
Q21: Give the formal charge on the phosphorus
Q22: Assume that the structure of BF<sub>3</sub> (boron
Q24: What is the C-N-O bond angle in
Q25: Which atomic orbital has two nodes?<br>A) 2p<br>B)
Q26: Consider this structure:<br> <span class="ql-formula"