Short Answer
Give the formal charge on the phosphorus atom in this structure. (Phosphorus is in group 5A directly under nitrogen in the periodic table.)
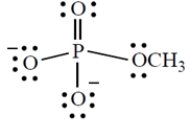
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: What is the geometry of the borohydride
Q17: Complete the structure for the cyanate ion
Q18: Given that the acetate anion has the
Q19: Add valence electrons to the structures so
Q20: Which compound has the largest dipole moment?<br><img
Q22: Assume that the structure of BF<sub>3</sub> (boron
Q23: Consider this compound:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Consider this
Q24: What is the C-N-O bond angle in
Q25: Which atomic orbital has two nodes?<br>A) 2p<br>B)
Q26: Consider this structure:<br> <span class="ql-formula"