Multiple Choice
Under which set of conditions must the following reaction always proceed to the right in order to reach equilibrium?
H2(g) + I2(g) 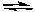 2 HI(g)
2 HI(g)
A) Qc < 1
B) Qc > 1
C) Qc = 1
D) Qc > Kc
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q7: What is the concentration in moles per
Q8: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q9: Calculate the concentrations of Cl<sub>2</sub> and ClF<sub>3</sub>
Q10: In which of the following will the
Q11: If the equilibrium constant for the following
Q13: Which is the correct equilibrium constant expression
Q14: Hidden Assumptions that make Equilibrium Calculations Easier
Q15: Calculate the COCl<sub>2</sub>, CO, and Cl<sub>2</sub> concentrations
Q16: Which of the following factors will
Q17: For the following reaction:<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg"