Multiple Choice
What would happen if Cl2 is added to the following system at equilibrium?
H2(g) + Cl2(g) 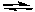 2 HCl(g)
2 HCl(g)
A) The concentrations of both H2 and HCl would remain the same.
B) The concentrations of both H2 and HCl would increase.
C) The concentrations of both H2 and HCl would decrease.
D) The concentration of H2 would increase, but HCl would decrease.
E) The concentration of H2 would decrease, but HCl would increase.
Correct Answer:

Verified
Correct Answer:
Verified
Q26: What is the fluoride ion concentration in
Q27: What is the relationship between the changes
Q28: If 6.5 x 10 <sup>-</sup> <sup>5</sup> moles
Q29: What is the correct solubility constant expression
Q30: One of the products of the reaction
Q32: Which of the following changes will
Q33: For the reaction<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="For
Q34: The equilibrium constant for the following reaction
Q35: If at any moment in time, we
Q36: Which of the following equations correctly describes