Multiple Choice
If at any moment in time, we find that Qc is larger than Kc for the reaction
Cl2(g) + 3 F2(g) 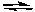 2 ClF3(g)
2 ClF3(g)
We can conclude that:
A) The reaction is at equilibrium.
B) The reaction cannot reach equilibrium.
C) The reaction must shift to the right to reach equilibrium.
D) The reaction must shift to the left to reach equilibrium.
E) None of the above are possible.
Correct Answer:

Verified
Correct Answer:
Verified
Q30: One of the products of the reaction
Q31: What would happen if Cl<sub>2</sub> is added
Q32: Which of the following changes will
Q33: For the reaction<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="For
Q34: The equilibrium constant for the following reaction
Q36: Which of the following equations correctly describes
Q37: What would happen to the extent of
Q38: Hidden Assumptions that make Equilibrium Calculations Easier
Q39: Hidden Assumptions that make Equilibrium Calculations
Q40: Hidden Assumptions that make Equilibrium Calculations Easier