Multiple Choice
The equilibrium constant for the following reaction becomes larger as the temperature at which the reaction is run increases.
2 NOCl(g) 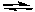 2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 at 400 K
2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 at 400 K
What does this tell us about the reaction?
A) Heat is given off in the reaction.
B) Heat is absorbed in the reaction.
C) Heat is neither given off nor absorbed in the reaction.
D) Increasing the amount of NOCl would also increase the equilibrium constant for the reaction.
E) Increasing the amount of NO would also increase the equilibrium constant for the reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q29: What is the correct solubility constant expression
Q30: One of the products of the reaction
Q31: What would happen if Cl<sub>2</sub> is added
Q32: Which of the following changes will
Q33: For the reaction<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="For
Q35: If at any moment in time, we
Q36: Which of the following equations correctly describes
Q37: What would happen to the extent of
Q38: Hidden Assumptions that make Equilibrium Calculations Easier
Q39: Hidden Assumptions that make Equilibrium Calculations