Multiple Choice
Which of the following changes will shift the following equilibrium to the right?
AgCl(s) 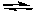 Ag+(aq) + Cl-(aq) H = 65.4 kJ/molrxn
Ag+(aq) + Cl-(aq) H = 65.4 kJ/molrxn
A) adding more AgCl(s)
B) adding NaCl
C) adding AgNO3
D) increasing the temperature
E) None of the above will shift the equilibrium to the right.
Correct Answer:

Verified
Correct Answer:
Verified
Q27: What is the relationship between the changes
Q28: If 6.5 x 10 <sup>-</sup> <sup>5</sup> moles
Q29: What is the correct solubility constant expression
Q30: One of the products of the reaction
Q31: What would happen if Cl<sub>2</sub> is added
Q33: For the reaction<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="For
Q34: The equilibrium constant for the following reaction
Q35: If at any moment in time, we
Q36: Which of the following equations correctly describes
Q37: What would happen to the extent of