Multiple Choice
Which of the following statements correctly describes the following reaction?
2 NH3(g) 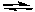 N2(g) + 3 H2(g) H = 92.2 kJ
N2(g) + 3 H2(g) H = 92.2 kJ
A) An increase in either pressure or temperature shifts the reaction to the left.
B) If the pressure of N2 is doubled and a new equilibrium established, the concentration of H2 at equilibrium will be reduced.
C) More ammonia will be present in the equilibrium mixture if a catalyst is added.
D) none of the above
E) all of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Hidden Assumptions that make Equilibrium Calculations
Q40: Hidden Assumptions that make Equilibrium Calculations Easier
Q41: The addition of I<sub>2</sub>(g) to a fixed
Q42: A solution is prepared in which the
Q43: Which of the following equations correctly describes
Q45: A solution is 0.10 M in Ca(NO<sub>3</sub>)<sub>2</sub>.
Q46: What would be the effect of decreasing
Q47: Assume that the reaction quotient, Q<sub>c</sub>, for
Q48: What is the K<sub>sp</sub> of PbBr<sub>2</sub> if
Q49: What would happen if O<sub>2</sub> were removed