Multiple Choice
The addition of I2(g) to a fixed volume container with I2(g) , H2(g) , and HI(g) at equilibrium at a given temperature would cause
H2(g) + I2(g) 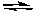 2 HI(g)
2 HI(g)
A) the concentrations of both H2 and HI to decrease.
B) the concentrations of both H2 and HI to increase.
C) the concentration of H2 to increase and HI to decrease.
D) the concentration of H2 to decrease and HI to increase.
E) Not enough information is given to determine how the concentrations will change.
Correct Answer:

Verified
Correct Answer:
Verified
Q36: Which of the following equations correctly describes
Q37: What would happen to the extent of
Q38: Hidden Assumptions that make Equilibrium Calculations Easier
Q39: Hidden Assumptions that make Equilibrium Calculations
Q40: Hidden Assumptions that make Equilibrium Calculations Easier
Q42: A solution is prepared in which the
Q43: Which of the following equations correctly describes
Q44: Which of the following statements correctly
Q45: A solution is 0.10 M in Ca(NO<sub>3</sub>)<sub>2</sub>.
Q46: What would be the effect of decreasing