Multiple Choice
What would be the effect of decreasing the pressure on the following reaction after it reaches equilibrium?
2 NO2(g) 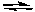 2 NO(g) + O2(g)
2 NO(g) + O2(g)
A) The NO and O2 concentrations would increase.
B) The NO and O2 concentrations would decrease.
C) The NO concentration would increase but O2 would decrease.
D) The O2 concentration would increase but NO would decrease.
E) The concentrations of NO and O2 would remain constant.
Correct Answer:

Verified
Correct Answer:
Verified
Q41: The addition of I<sub>2</sub>(g) to a fixed
Q42: A solution is prepared in which the
Q43: Which of the following equations correctly describes
Q44: Which of the following statements correctly
Q45: A solution is 0.10 M in Ca(NO<sub>3</sub>)<sub>2</sub>.
Q47: Assume that the reaction quotient, Q<sub>c</sub>, for
Q48: What is the K<sub>sp</sub> of PbBr<sub>2</sub> if
Q49: What would happen if O<sub>2</sub> were removed
Q50: Of the compounds in the table
Q51: Which one of the following graphs best