Multiple Choice
Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g) 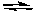 2H2(g) + O2(g)
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-Starting with only 0.10 M H2O(g) , which equation correctly shows the equilibrium concentrations of the reactants and products? ( C represents the change in concentration of O2(g) )
A) 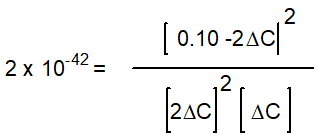
B) 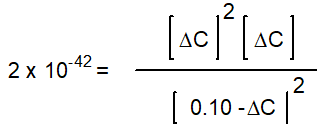
C) 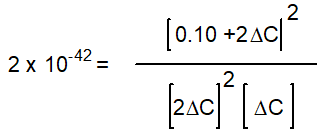
D) 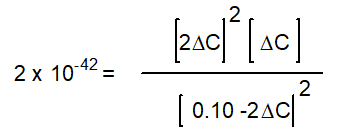
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q34: The equilibrium constant for the following reaction
Q35: If at any moment in time, we
Q36: Which of the following equations correctly describes
Q37: What would happen to the extent of
Q38: Hidden Assumptions that make Equilibrium Calculations Easier
Q40: Hidden Assumptions that make Equilibrium Calculations Easier
Q41: The addition of I<sub>2</sub>(g) to a fixed
Q42: A solution is prepared in which the
Q43: Which of the following equations correctly describes
Q44: Which of the following statements correctly