Multiple Choice
What is the effect of increasing the temperature on the following reaction?
2 CO(g) + O2(g) 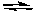 2 CO2(g) + heat
2 CO2(g) + heat
A) The equilibrium will shift to the left.
B) The equilibrium will shift to the right.
C) The partial pressure of CO2 will increase.
D) The partial pressure of O2 will decrease.
E) It will have no effect.
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Which of the following graphs best represents
Q20: Assume that the equilibrium constant for the
Q21: A 50.0 mL solution of 0.015 M
Q22: Which is the correct reaction for the
Q23: Which of the following statements is correct?<br>A)
Q25: Suppose that the reaction quotient, Q<sub>c</sub>,
Q26: What is the fluoride ion concentration in
Q27: What is the relationship between the changes
Q28: If 6.5 x 10 <sup>-</sup> <sup>5</sup> moles
Q29: What is the correct solubility constant expression