Multiple Choice
Assume that the equilibrium constant for the following reaction is known.
2 NO2(g) 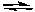 2 NO(g) + O2(g) K1 = 7.4 x 10-16 (at 25°C)
2 NO(g) + O2(g) K1 = 7.4 x 10-16 (at 25°C)
What is the correct value of the equilibrium constant for the opposite reaction?
2 NO(g) + O2(g) 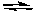 2 NO2(g) K2 = ?
2 NO2(g) K2 = ?
A) K2 = K1 = 7.4 x 10-16
B) K2 = 1/K1 = 1.4 x 1015
C) K2 = K1(RT) = 1.8 x 10-14
D) K2 = K1(RT) -1 = 3.0 x 10-17
E) impossible to determine from this information
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Calculate the COCl<sub>2</sub>, CO, and Cl<sub>2</sub> concentrations
Q16: Which of the following factors will
Q17: For the following reaction:<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg"
Q18: Based on the information given in the
Q19: Which of the following graphs best represents
Q21: A 50.0 mL solution of 0.015 M
Q22: Which is the correct reaction for the
Q23: Which of the following statements is correct?<br>A)
Q24: What is the effect of increasing the
Q25: Suppose that the reaction quotient, Q<sub>c</sub>,