Multiple Choice
Which is the correct reaction for the solubility constant expression (Ksp) of CaF2(s) ?
A) 2F-(aq) + Ca2+(aq) 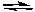 CaF2(s)
CaF2(s)
B) 2F(aq) + Ca (aq) 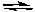 CaF2(s)
CaF2(s)
C) CaF2(s) 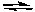 CaF2(g)
CaF2(g)
D) CaF2(s) 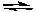 Ca(g) + 2F(g)
Ca(g) + 2F(g)
E) CaF2(s) 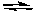 Ca2+(aq) + 2F-(aq)
Ca2+(aq) + 2F-(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q17: For the following reaction:<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg"
Q18: Based on the information given in the
Q19: Which of the following graphs best represents
Q20: Assume that the equilibrium constant for the
Q21: A 50.0 mL solution of 0.015 M
Q23: Which of the following statements is correct?<br>A)
Q24: What is the effect of increasing the
Q25: Suppose that the reaction quotient, Q<sub>c</sub>,
Q26: What is the fluoride ion concentration in
Q27: What is the relationship between the changes